- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记4.1.3 Determining Concentrations
Core Practical 3: Hydrochloric Acid Concentration
Performing the Titration
- The key piece of equipment used in the titration is the burette
- Burettes are usually marked to a precision of 0.10 cm3
- Since they are analogue instruments, the uncertainty is recorded to half the smallest marking, in other words to ±0.05 cm3
- The end point or equivalence point occurs when the two solutions have reacted completely and is shown with the use of an indicator
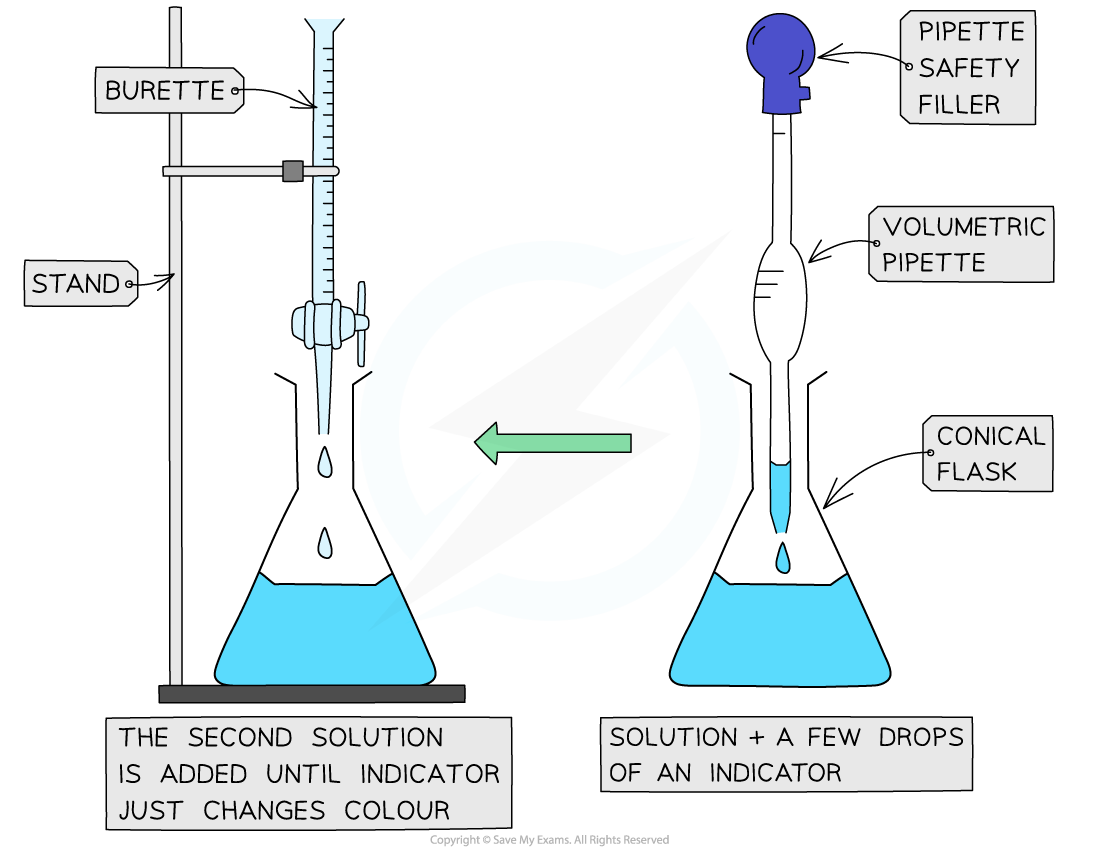
The steps in a titration
- A white tile is placed under the conical flask while the titration is performed, to make it easier to see the colour change
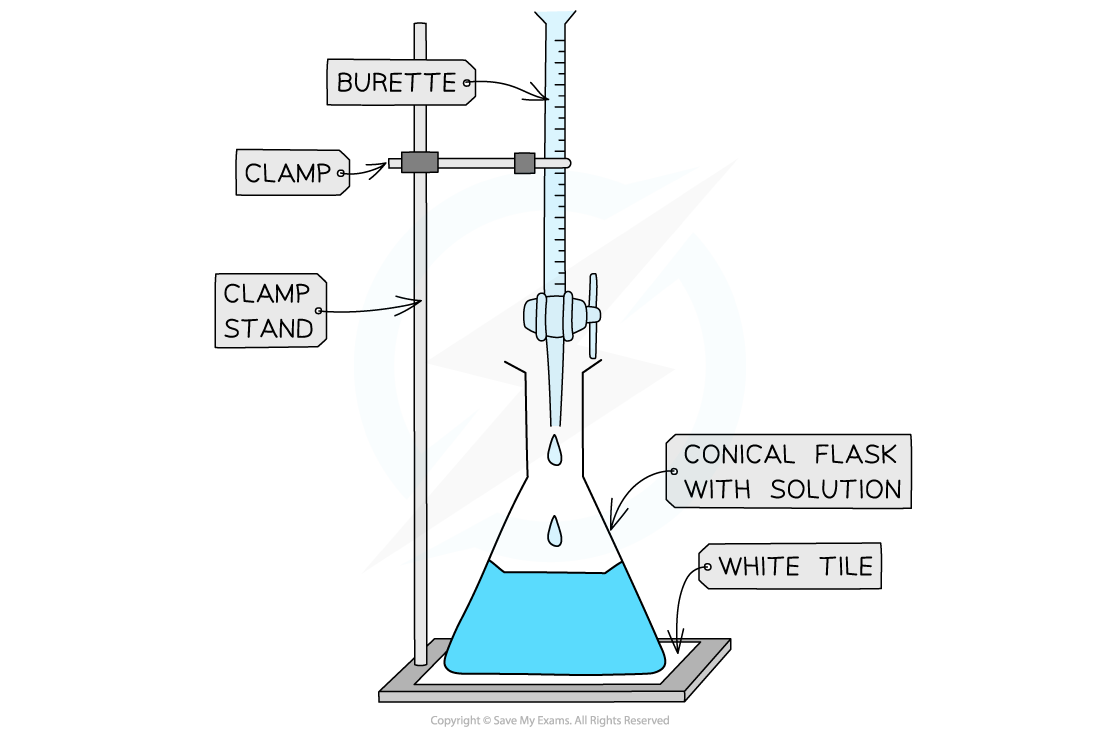
Titrating
- The steps in a titration are:
- Measuring a known volume (usually 20 or 25 cm3) of one of the solutions with a volumetric pipette and placing it into a conical flask
- The other solution is placed in the burette
- To start with, the burette will usually be filled to 0.00 cm3
- A few drops of the indicator are added to the solution in the conical flask
- The tap on the burette is carefully opened and the solution added, portion by portion, to the conical flask until the indicator starts to change colour
- As you start getting near to the end point, the flow of the burette should be slowed right down so that the solution is added dropwise
- You should be able to close the tap on the burette after one drop has caused the colour change
- Multiple runs are carried out until concordant results are obtained
- Concordant results are within 0.1 cm3 of each other
Recording and processing titration results
- Both the initial and final burette readings should be recorded and shown to a precision of ±0.05 cm3, the same as the uncertainty
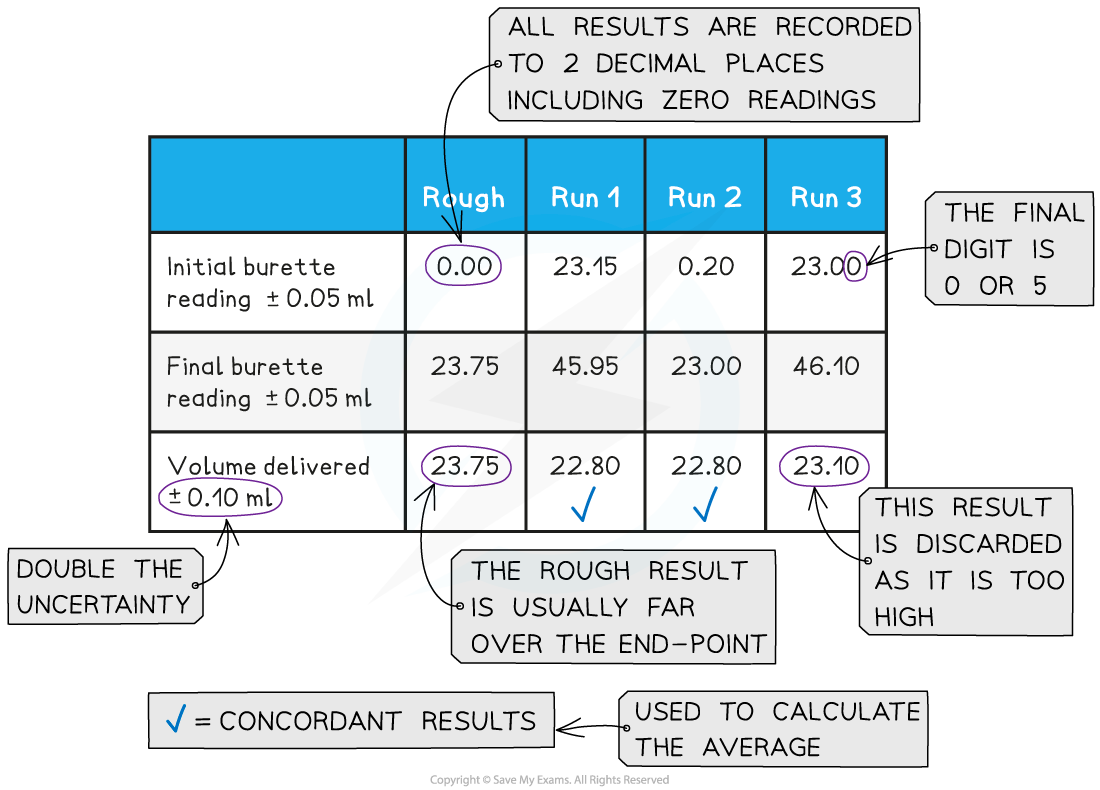
A typical layout and set of titration results
- The volume delivered (titre) is calculated and recorded to an uncertainty of ±0.10 cm3
- The uncertainty is doubled, because two burette readings are made to obtain the titre (V final – V initial), following the rules for propagation of uncertainties
- Concordant results are then averaged, and non-concordant results are discarded
- The appropriate calculations are then done
Worked Example
25.0 cm3 of hydrochloric acid was titrated with a 0.200 mol dm-3 solution of sodium hydrogencarbonate, NaHCO3.
NaHCO3 + HCl → NaCl + H2O + CO2
Use the following results to calculate the concentration of the acid, to 3 significant figures.
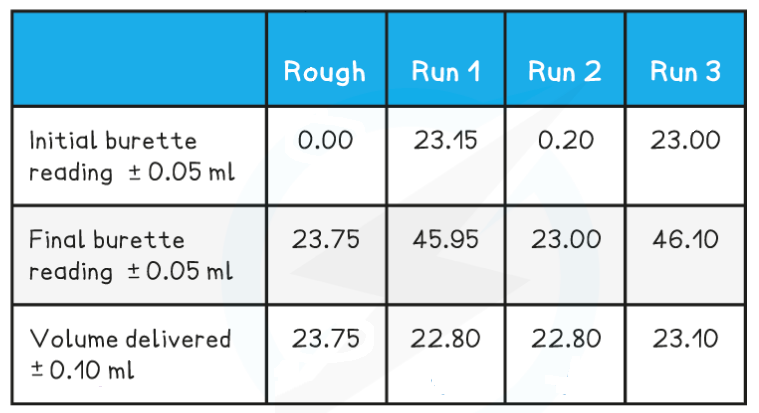
Answer
Step 1: Calculate the average titre
-
- Average titre
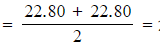 22.80 cm3
22.80 cm3
- Average titre
Step 2: Calculate the number of moles of sodium hydrogencarbonate
-
- Moles
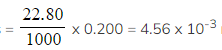 moles
moles
- Moles
Step 3: Calculate (or deduce) the number of moles of hydrochloric acid
-
- The stoichiometry of NaHCO3 : HCl is 1 : 1
- Therefore, the number of moles of sodium hydrogencarbonate is also 4.56 x 10-3 moles
Step 4: Calculate the concentration of hydrochloric acid
-
- Concentration
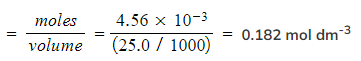
- Concentration
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









