- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记3.5.2 Reactions of Alcohols
Reactions of Alcohols
Combustion of alcohols
- Alcohols react with oxygen in the air when ignited and undergo complete combustion to form carbon dioxide and water
alcohol + oxygen → carbon dioxide + water

Complete combustion of alcohols to produce carbon dioxide and water
Conversions to halogenoalkanes
- These reactions involve replacing the hydroxyl group in an alcohol molecule with a halogen atom(known as halogenation)
- Different methods are required for each halogen
Chlorination
- Phosphorus(V) chloride is added to the alcohol resulting in a vigorous reaction at room temperature
- This means the mixture doesn't need heating
- This reaction also produces two inorganic products: phosphoryl chloride and hydrogen chloride
CH3CH2CH2OH + PCl5 → CH3CH2CH2Cl + POCl3 + HCl
- Chlorination of tertiary alcohols can be carried out in a different way by mixing (shaking) with hydrochloric acid at room temperature
- An example equation for the reaction of 2-methyl propan-2-ol is:
(CH3)3COH + HCl → (CH3)3CCl + H2O
- This reaction does not work for primary and secondary alcohols
Bromination
- This reaction is carried out using a warmed mixture of potassium bromide and 50% concentrated sulfuric acid with the reacting alcohol
- More concentrated sulfuric acid would oxidise bromide ions to bromine resulting in different products
- The reaction can be written as two equations as the inorganic reactants first react together to form hydrogen bromide and potassium sulfate
2KBr + H2SO4 → K2SO4 +2HBr
- The resulting hydrogen bromide then reacts with the alcohol, for example the reaction with butan-1-ol would be as follows:
CH3CH2CH2CH2OH + HBr → CH3CH2CH2CH2Br + H2O
Iodination
- This reaction is carried out using a mixture of red phosphorus and iodine with the alcohol whilst heating under reflux
- Similar to bromination, the reaction can be written as two equations as the inorganic reactants first react to form phosphorus(III) iodide
2P + 3I2 → 2PI3
- The reaction for the iodination of ethanol would be:
3C2H5OH + PI3 → 3C2H5I + H3PO3
- This reaction results in the formation of phosphoric acid as shown above
Dehydration to Alkenes
- Dehydration is done by heating the alcohol with concentrated phosphoric acid
- The reaction is similar to the elimination reaction of a halogenoalkene
- The OH group and hydrogen of adjacent carbons are removed forming a C=C bond
- The equation for the dehydration of ethanol would be
CH3CH2OH → CH2=CH2 + H2O
- Phosphoric acid does not appear in the equation as the water formed dilutes the concentrated phosphoric acid
Oxidation of alcohols
- Primary alcohols can be oxidised to form aldehydes which can undergo further oxidation to form carboxylic acids
- Secondary alcohols can be oxidised to form ketones only
- Tertiary alcohols do not undergo oxidation
- The oxidising agents of alcohols include acidified K2Cr2O7
- Acidified potassium dichromate(VI), K2Cr2O7, is an orange oxidising agent
- Acidified means that that the potassium dichromate(VI) is in a solution of dilute acid (such as dilute sulfuric acid)
- For potassium dichromate(VI) to act as an oxidising agent, it itself needs to be reduced
- This reduction requires hydrogen (H+) ions which are provided by the acidic medium
- When alcohols are oxidised the orange dichromate ions (Cr2O72-) are reduced to green Cr3+ ions
- The primary alcohol is added to the oxidising agent and warmed
- The aldehyde product has a lower boiling point than the alcohol reactant so it can be distilled off as soon as it forms
- If the aldehyde is not distilled off, further refluxing with excess oxidising agent will oxidise it to a carboxylic acid
- Since ketones cannot be further oxidised, the ketone product does not need to be distilled off straight away after it has been formed
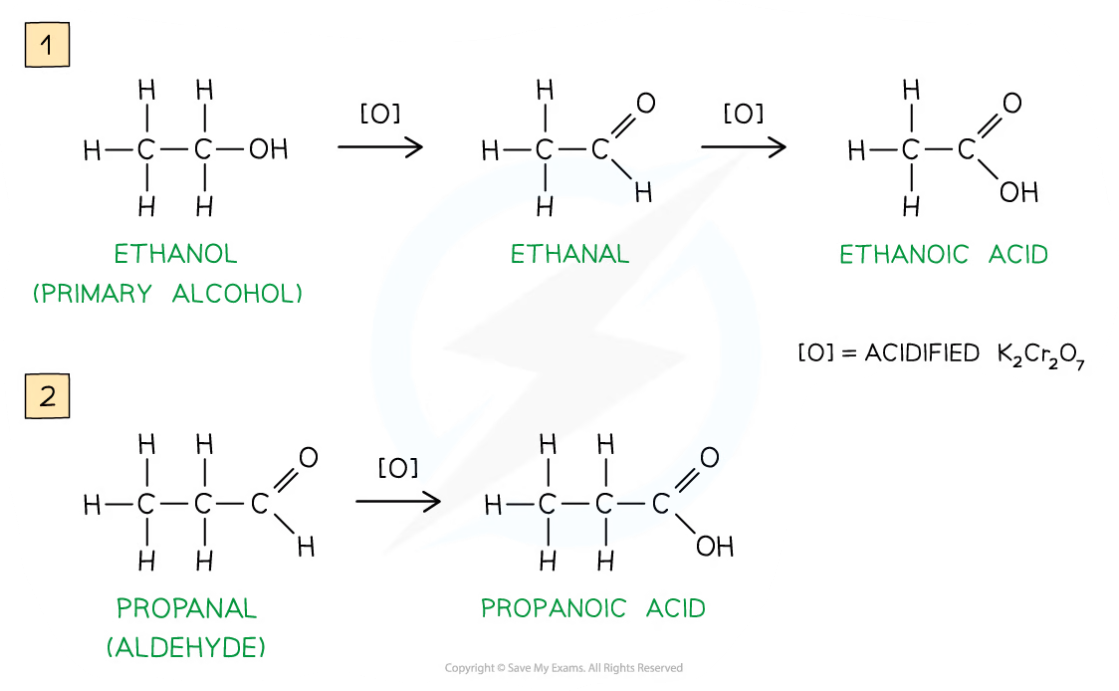
Oxidation Stages of Primary Alcohols

Oxidation of propan-2-ol by acidified K2Cr2O7 to form a ketone
- The presence of an aldehyde group (-CHO) in an unknown compound can be determined by the oxidising agents Fehling’s and Tollens’ reagents
Fehling’s solution
- Fehling’s solution is an alkaline solution containing copper(II) ions which act as the oxidising agent
- When warmed with an aldehyde, the aldehyde is oxidised to a carboxylic acid and the Cu2+ ions are reduced to Cu+ ions
- In the alkaline conditions, the carboxylic acid formed will be neutralised to a carboxylate ion (the -COOH will lose a proton to become -COO- )
- The carboxylate ion (-COO-) will form a salt with a positively charged metal ion such as sodium (-COO-Na+)
- The clear blue colour of the solution turns opaque red due to the formation of a copper(I) oxide precipitate
- Ketones cannot be oxidised and therefore give a negative test when warmed with Fehling’s solution
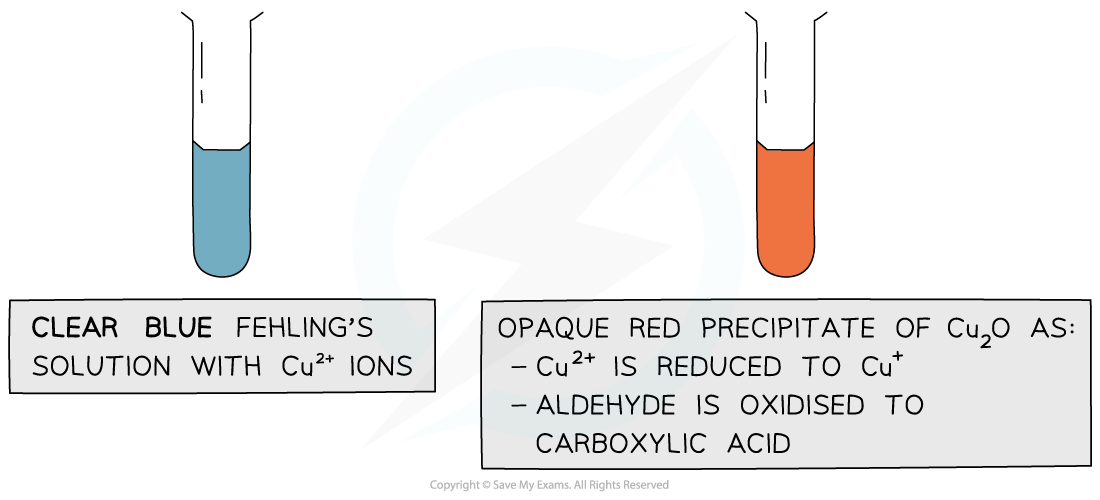
The copper(II) ions in Fehling’s solution are oxidising agents, oxidising the aldehyde to a carboxylic acid and getting reduced themselves to copper(I) ions in the Cu2O precipitate
Tollens’ reagent
- Tollens' reagent is an aqueous alkaline solution of silver nitrate in excess ammonia solution
- Tollen’s reagent is also called ammoniacal silver nitrate solution
- When warmed with an aldehyde, the aldehyde is oxidised to a carboxylic acid and the Ag+ ions are reduced to Ag atoms
- In the alkaline conditions, the carboxylic acid will become a carboxylate ion and form a salt
- The Ag atoms form a silver ‘mirror’ on the inside of the tube
- Ketones cannot be oxidised and therefore give a negative test when warmed with Tollens’ reagent
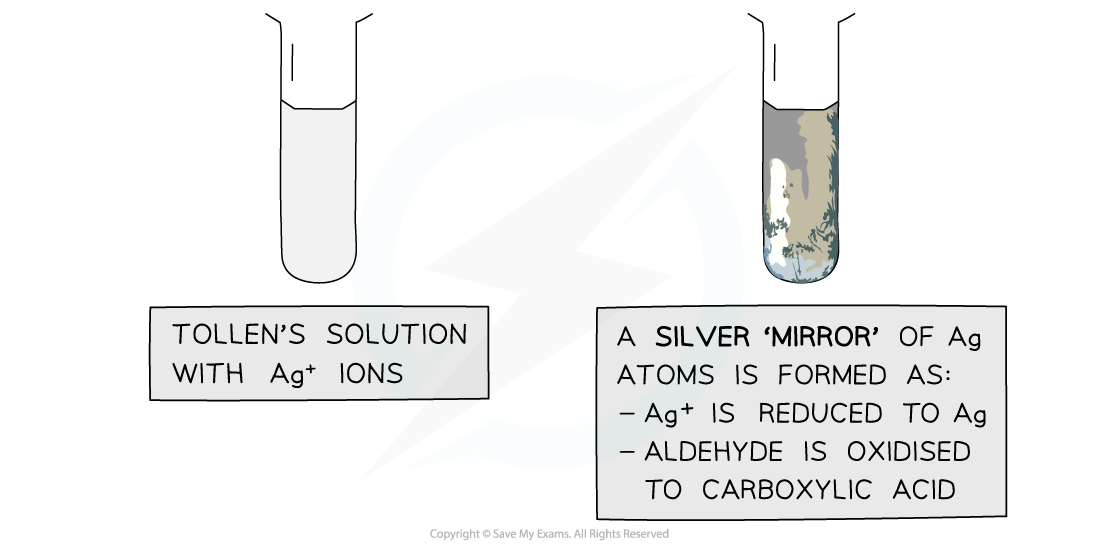
The Ag+ ions in Tollens’ reagent are oxidising agents, oxidising the aldehyde to a carboxylic acid and getting reduced themselves to silver atoms
Different practical techniques
- Because of the easier oxidation of aldehydes compared to alcohols, two different techniques are used
- Heating under reflux
- Distillation with addition
Heating under reflux
- This technique is used when we want full oxidation
- Producing a carboxylic acid for a primary alcohol
- Producing a ketone for a secondary alcohol
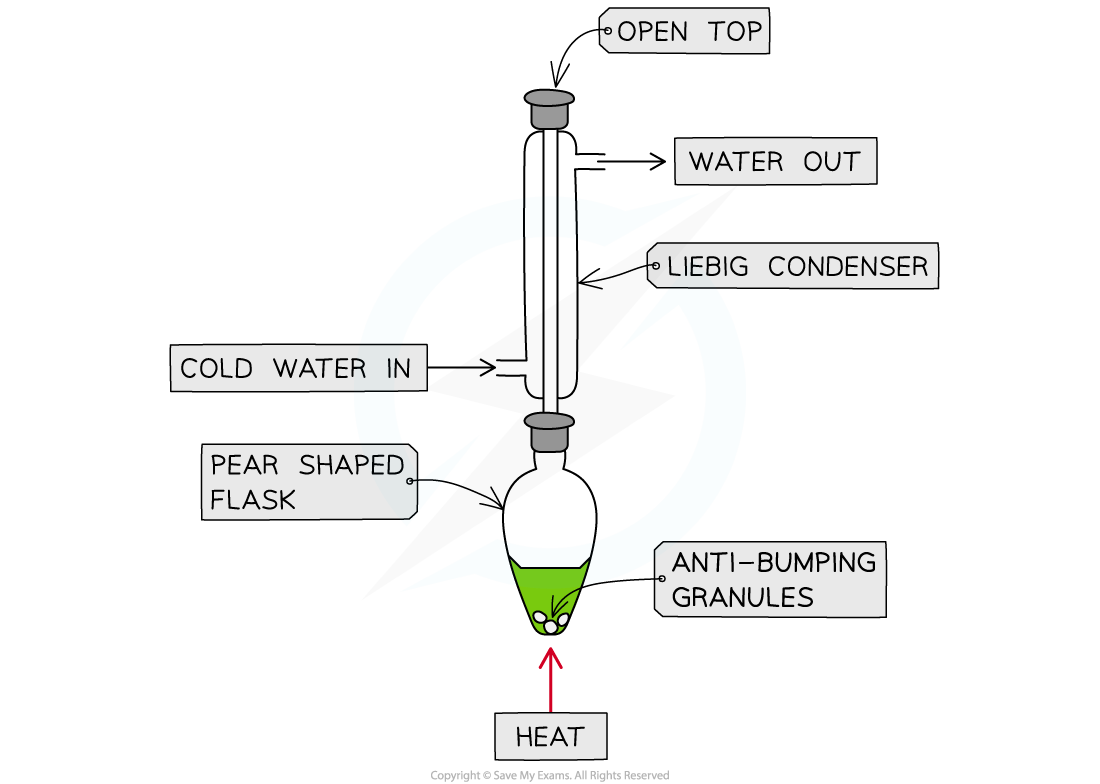
Apparatus set up for heating under reflux
- This set up means any products of oxidation remain in the reaction mixture
- Products which boil off condense in the vertical condenser then return to the heating flask
Distillation with addition
- This technique is used when we do not want to complete oxidation
- To obtain an aldehyde rather than carboxylic acid for primary alcohol
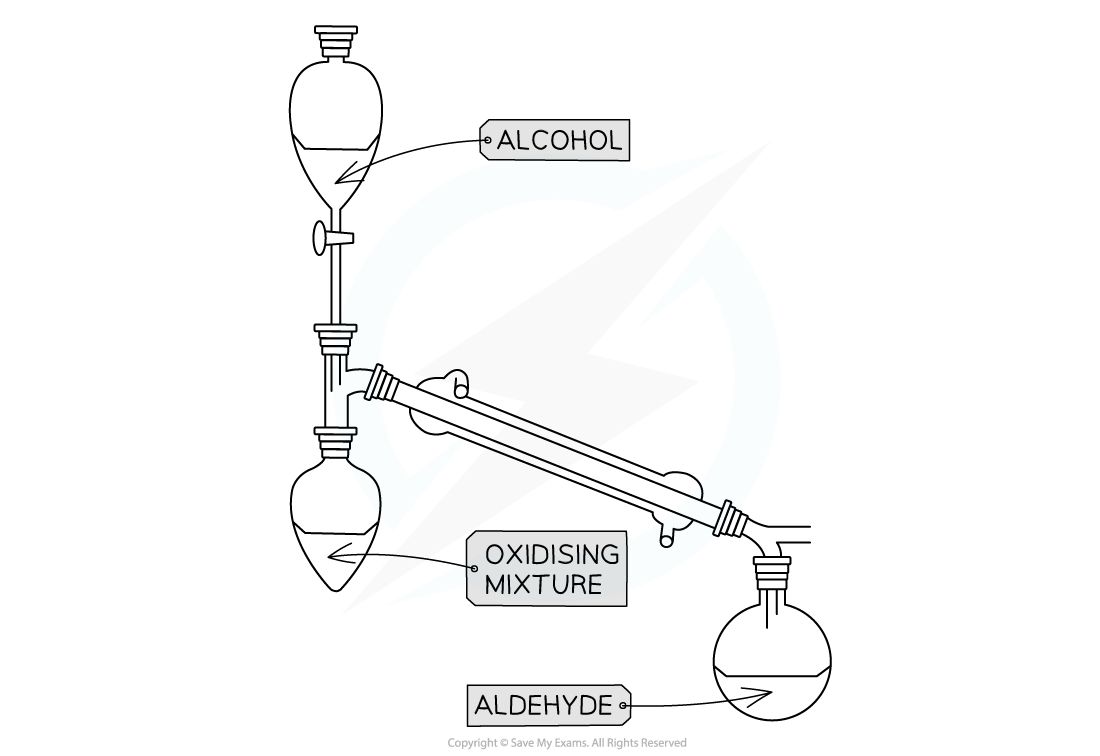
- To obtain an aldehyde rather than carboxylic acid for primary alcohol
Apparatus set up for distillation with addition
- Only the oxidising agent is heated whilst the alcohol is slowly added
- When the aldehyde is formed it immediately distils off as it has a much lower boiling point than the alcohol used to make it
- The aldehyde is then collected in the reciever
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









