- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记3.4.5 Reactivity of Halogenoalkanes
Reactivity of Halogenoalkanes
- Nucleophilic substitution reactions can occur in two different ways (known as SN2 and SN1 reactions) depending on the structure of the halogenoalkane involved
- Tertiary halogenoalkanes favour SN2 reactions
- Primary halogenoalkanes favour SN1 reactions
SN1 reactions
- In tertiary halogenoalkanes, the carbon that is attached to the halogen is also bonded to three alkyl groups
- These halogenoalkanes undergo nucleophilic substitution by an SN1 mechanism
- ‘S’ stands for ‘substitution’
- ‘N’ stands for ‘nucleophilic’
- ‘1’ means that the rate of the reaction (which is determined by the slowest step of the reaction) depends on the concentration of only one reagent, the halogenoalkane

- The SN1 mechanism is a two-step reaction
- In the first step, the C-X bond breaks heterolytically and the halogen leaves the halogenoalkane as an X- ion (this is the slow and rate-determining step)
- This forms a tertiary carbocation (which is a tertiary carbon atom with a positive charge)
- In the second step, the tertiary carbocation is attacked by the nucleophile
- For example, the nucleophilic substitution of 2-bromo-2-methylpropane by hydroxide ions to form 2-methyl-2-propanol
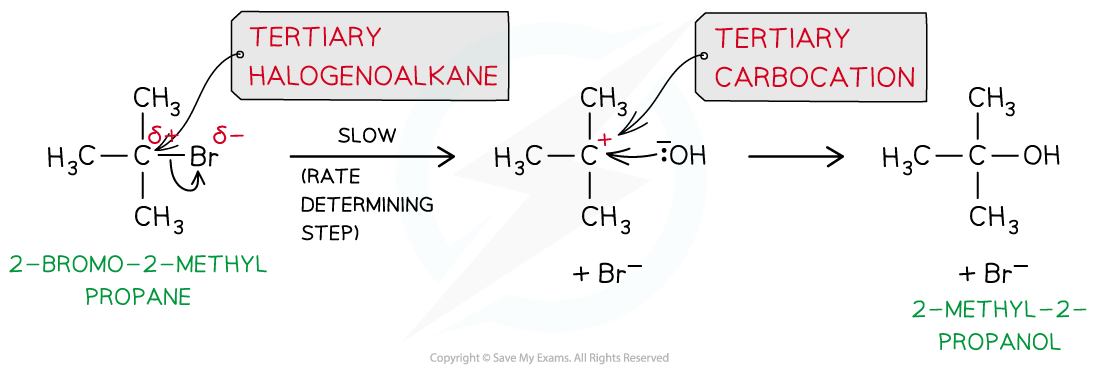
The mechanism of nucleophilic substitution in 2-bromo-2-methylpropane which is a tertiary halogenoalkane
SN2 reactions
- In primary halogenoalkanes, the carbon that is attached to the halogen is bonded to one alkyl group
- These halogenoalkanes undergo nucleophilic substitution by an SN2 mechanism
- ‘S’ stands for ‘substitution’
- ‘N’ stands for ‘nucleophilic’
- ‘2’ means that the rate of the reaction (which is determined by the slowest step of the reaction) depends on the concentration of both the halogenoalkane and the nucleophile ions

- The SN2 mechanism is a one-step reaction
- The nucleophile donates a pair of electrons to the δ+ carbon atom of the halogenoalkane to form a new bond
At the same time, the C-X bond is breaking and the halogen (X) takes both electrons in the bond
- The halogen leaves the halogenoalkane as an X- ion
- The nucleophile donates a pair of electrons to the δ+ carbon atom of the halogenoalkane to form a new bond
- For example, the nucleophilic substitution of bromoethane by hydroxide ions to form ethanol
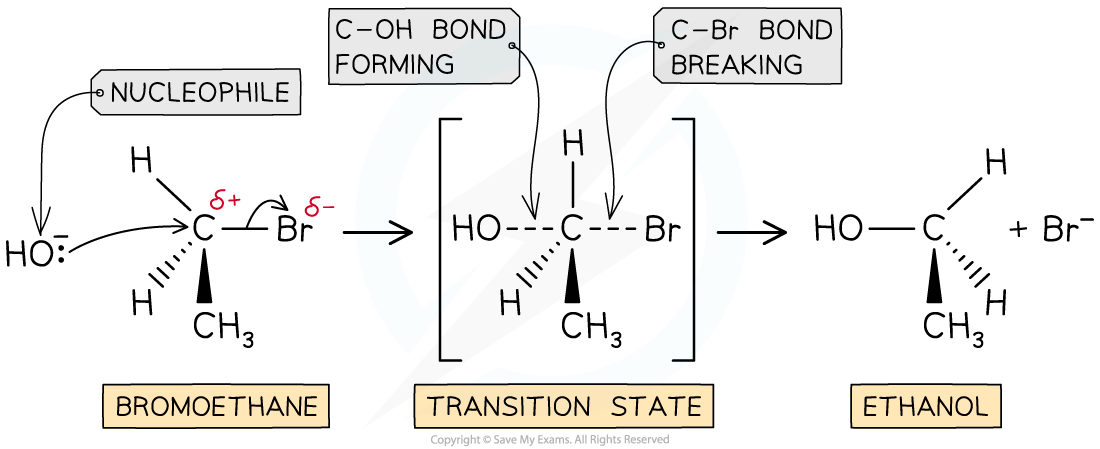
The SN2 mechanism of bromoethane with hydroxide causing an inversion of configuration
Bond Enthalpy & Halogenoalkane Reactivity
Bond Enthalpy
- The halogenoalkanes have different rates of substitution reactions
- Since substitution reactions involve breaking the carbon-halogen bond the bond energies can be used to explain their different reactivities
Halogenoalkane Bond Energy
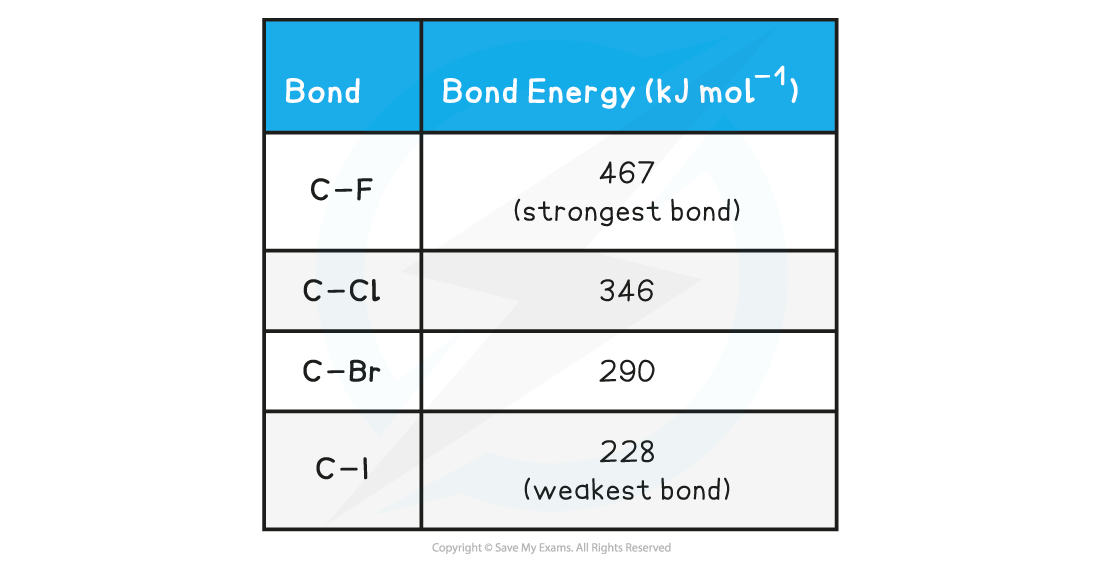
- The table above shows that the C-I bond requires the least energy to break, and is therefore the weakest carbon-halogen bond
- During substitution reactions, the C-I bond will, therefore, heterolytically break as follows:
R3C-I + OH- → R3C-OH + I-
halogenoalkane alcohol
- The C-F bond, on the other hand, requires the most energy to break and is, therefore, the strongest carbon-halogen bond
- Fluoroalkanes will, therefore, be less likely to undergo substitution reactions
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









