- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记2.2.5 Flame Tests
Flame Tests
- Metal ions produce a colour if heated strongly in a flame
- Ions from different metals produce different colours
- The flame test is thus used to identify metal ions by the colour of the flame they produce
- Dip the loop of an unreactive metal wire such as nichrome or platinum in concentrated acid, and then hold it in the blue flame of a Bunsen burner until there is no colour change
- This cleans the wire loop and avoids contamination
- This is an important step as the test will only work if there is just one type of ion present
- Two or more ions means the colours will mix, making identification erroneous
- Dip the loop into the solid sample and place it in the edge of the blue Bunsen flame
- Avoid letting the wire get so hot that it glows red otherwise this can be confused with a flame colour

Diagram showing the technique for carrying out a flame test
Explanation for the occurrence of the flame
- In a flame test the heat causes the electron to move to a higher energy level
- The electron is unstable at this energy level so falls back down
- As it drops back down from the higher to a lower energy level, energy is emitted in the form of visible light energy with the wavelength of the observed light
Colours observed in flame tests
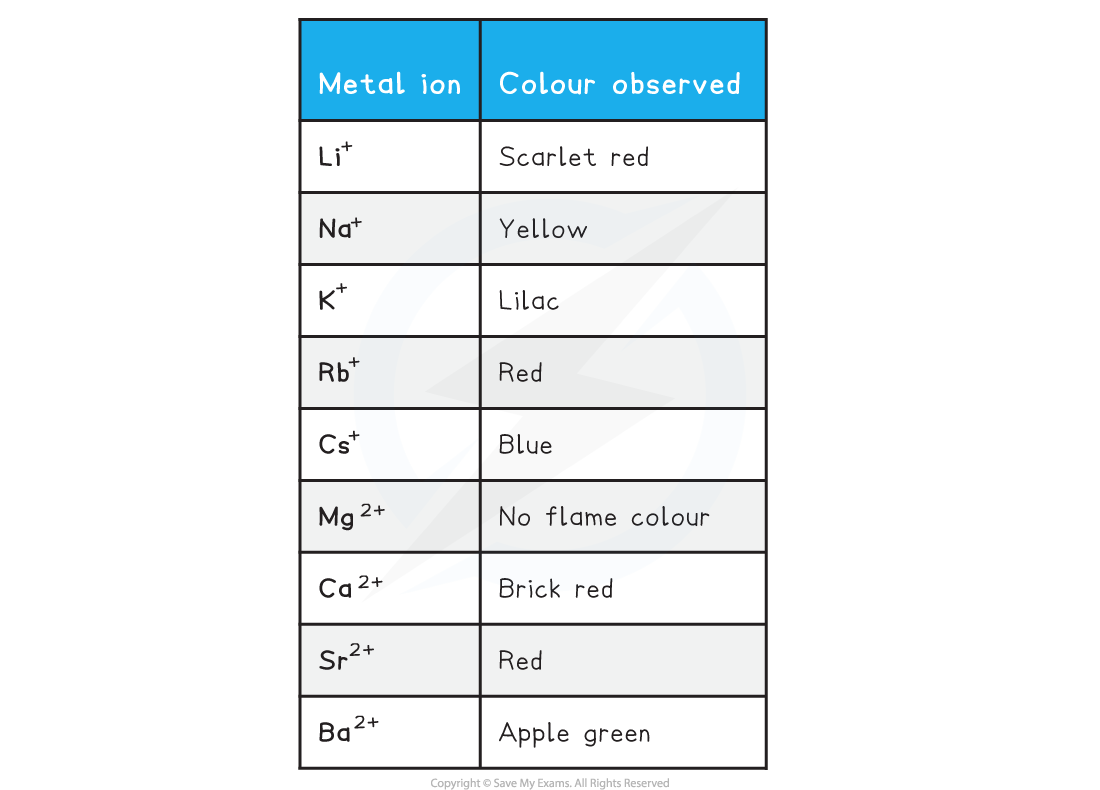
- Why does Mg2+ not have an observed colour?
- The energy emitted during a flame test involving magnesium is outside the visible spectrum
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









