- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记2.2.2 Reactions of Group 2
Group 2 Elements
Reactions with water and oxygen
- The reaction of group 2 metals with oxygen follows the following general equation:
2M (s) + O2 (g) → 2MO (s)
Where M is any metal in group 2
Remember than Sr and Ba also form a peroxide, MO2
- The reaction of all metals with water follows the following general equation:
M (s) + 2H2O (l) → M(OH)2 (s) + H2 (g)
Except for, Be which does not react with water
Group 2 Metals reacting with Water and with Oxygen - Equations
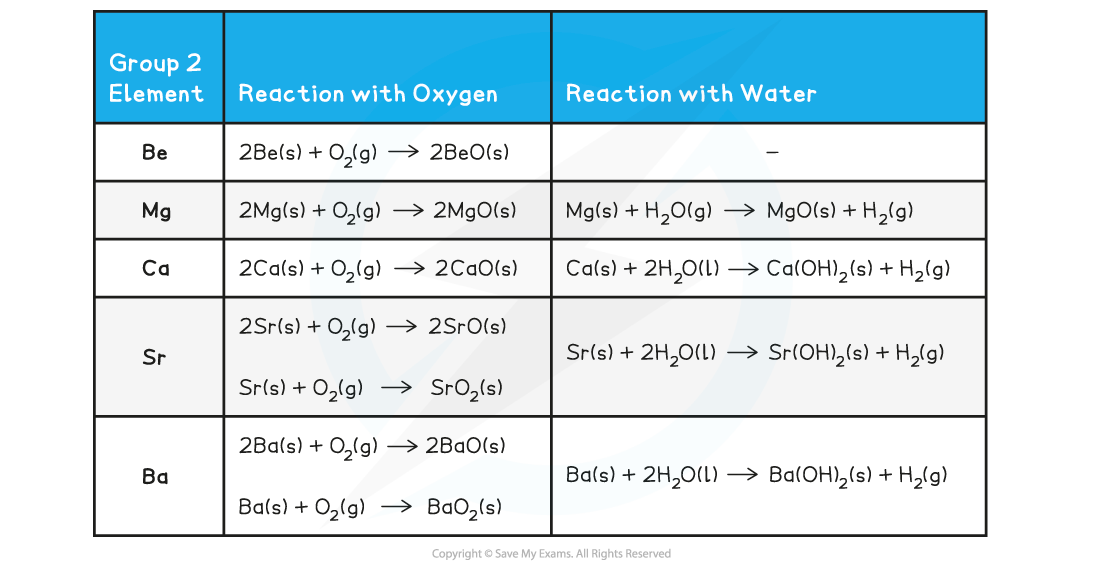
- Magnesium reacts extremely slowly with cold water:
Mg (s) + 2H2O (l) → Mg(OH)2 (aq) + H2 (g)
- The solution formed is weakly alkaline (pH 9-10) as magnesium hydroxide is only slightly soluble
- However, when magnesium is heated in steam, it reacts vigorously with steam to make magnesium oxide and hydrogen gas:
Mg (s) + H2O (g) → MgO (s) + H2 (g)
Reactions with chlorine
- Group 2 metals react with chlorine gas to give the metal chloride
- For example
Mg (s) + Cl2 (g) → MgCl2 (s)
Group 2 Oxides
Reactions of Group 2 oxides with water
- All Group 2 oxides are basic, except for BeO which is amphoteric (it can act both as an acid and base)
- Group 2 oxides react water to form alkaline solutions which get more alkaline going down the group
Group 2 Oxides reacting with Water
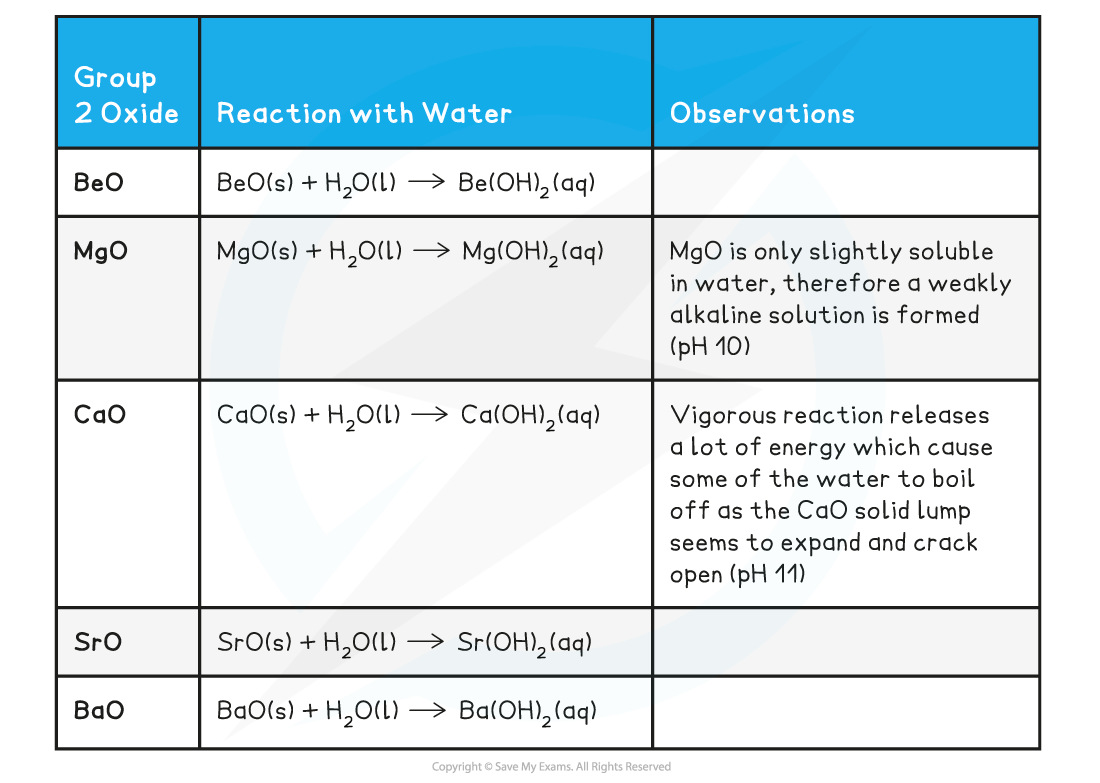
- Remember that:
oxide + water → hydroxide
- You should know that calcium hydroxide, when in solution, is also called limewater
Reactions of Group 2 oxides with acid
- Group 2 sulfates also form when a Group 2 oxide is reacted with sulfuric acid
- The insoluble sulfates form at the surface of the oxide, which means that the solid oxide beneath it can’t react with the acid
- This can be prevented to an extent by using the oxide in powder form and stirring, in which case neutralisation can take place
- Remember that:
oxide + dilute hydrochloric acid → chloride + water
oxide + dilute sulfuric acid → sulfate + water
Reactions of Group 2 hydroxides
- The Group 2 metal hydroxides form colourless solutions of metal salts when they react with a dilute acid
- The sulfates decrease in solubility going down the group (barium sulfate is an insoluble white precipitate)
Group 2 Hydroxide Reactions with Dilute Acids
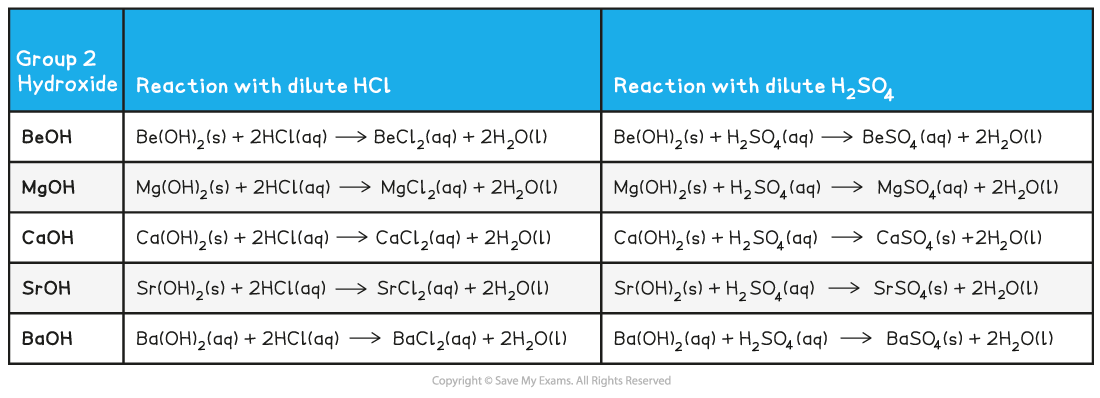
- Remember that:
hydroxide + dilute hydrochloric acid → chloride + water
hydroxide + dilute sulfuric acid → sulfate + water
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









