- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记2.2.1 Explaining Group 2 Trends
Ionisation Energy
Chemical trends
- All elements in Group 2 (also called alkali earth metals) have two electrons in their outermost principal quantum shell
- All Group 2 metals can form ionic compounds in which they donate these two outermost electrons (so they act as reducing agents) to become an ion with +2 charge (so they themselves become oxidised)
- Going down the group, the metals become more reactive
- This can be explained by looking at the Group 2 ionisation energies:
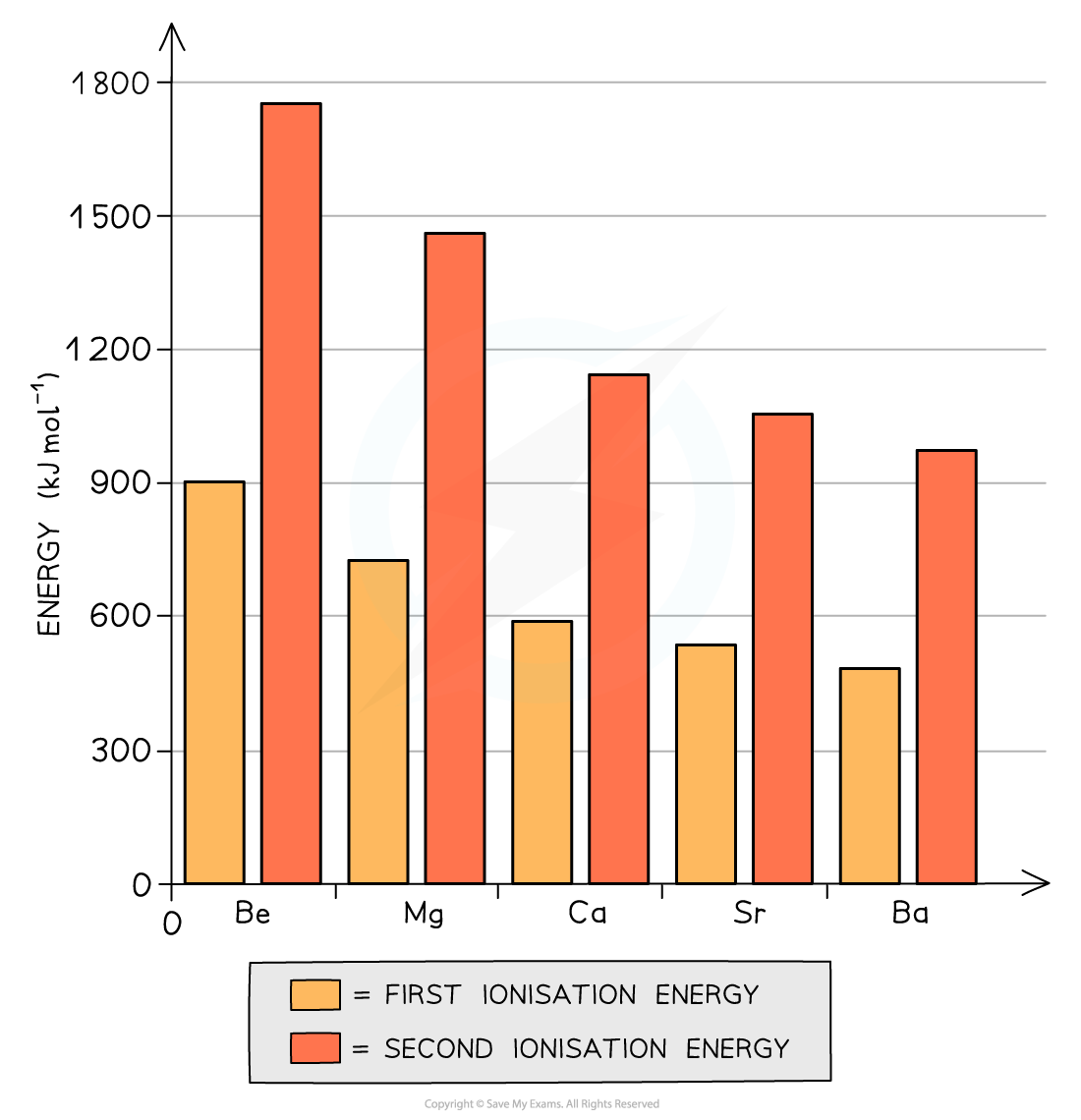
The graph shows that both the first and second ionization energies decrease going down the group
- The first ionisation energy is the energy needed to remove the first outer electron of an atom
- The second ionisation energy is the energy needed to remove the second outer electron of an atom
- The graph above shows that going down the group, it becomes easier to remove the outer two electrons of the metals
- Though the nuclear charge increases going down the group (because there are more protons), factors such as an increased shielding effect and a larger distance between the outermost electrons and nucleus outweigh the attraction of the higher nuclear charge
Reactivity
- As a result of the deceases in ionisation energy, the elements become more reactive going down the group as it gets easier for the atoms to lose two electrons and become 2+ ions
- This trend is shown by looking at reactions of the Group 2 metals:
- With dilute hydrochloric acid: bubbles of hydrogen gas are given off much faster indicating that the reactions become more vigorous
- For example:
Mg (s) + HCl (aq) → MgCl2 (aq) + H2 (g)
-
- With oxygen: the metals get more reactive with oxygen down the group (Ba is so reactive, that it must be stored in oil to prevent it from reacting with oxygen in air)
Mg (s) + O2 (g) → MgO (s)
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








