- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记2.1.4 Ionic Equations
Writing Chemical Formulae
- Oxidation numbers are a useful tool for naming compounds as some elements can exist with more than one oxidation number
- For compound with two elements it is straight forward to name the compound
- For example
- PCl3 is phosphorus(III) chloride or phosphorus trichloride
- PCl5 is phosphorus(V) chloride or phosphorus pentachloride
- OF2 is oxygen difluoride
- O2F2 is dioxygen difluoride
- In order to name a more complete compound we use Roman numerals for the element that has a variable oxidation number
- K2CrO4 potassium chromate(VI)
Worked Example
Can you name these metal compounds?
- Cu2O
- MnSO4
- Na2CrO4
- KMnO4
- Na2Cr2O7
Answer:
Answer 1: copper(I) oxide:
The ox. no. of 1 O atom is -2 and Cu2O has overall no charge so the ox. no. of Cu is +1
Answer 2: manganese(II) sulfate:
The charge on the sulfate ion is -2, so the charge on Mn and ox. no. is +2
Answer 3: sodium chromate(VI):
The ox. no. of 2 Na atoms is +2 so CrO4 has an overall -2 charge, so the ox. no. of Cr is +6
Answer 4: potassium manganate(VII):
The ox. no. of a K atom is +1 so MnO4 has overall -1 charge, so the ox. no. of Mn is +7
Answer 5: sodium dichromate(VI):
The ox. no. of 2 Na atoms is +2 so Cr2O7 has an overall -2 charge, so the ox. no. of Cr is +6. To distinguish it from CrO4 we use the prefix di in front of the anion
Ionic Half-Equations
Balancing full ionic equations
- Balancing equations using redox principles is a useful skill and is best illustrated by following an example
- It is important to follow a methodical step-by-step approach so that you don't get lost:
Worked Example
Writing overall redox reactions
Manganate(VII) ions (MnO4- ) react with Fe2+ ions in the presence of acid (H+) to form Mn2+ ions, Fe3+ ions and water
Write the overall redox equation for this reaction
Answer
Step 1: Write the unbalanced equation and identify the atoms which change in oxidation number

Step 2: Deduce the oxidation number changes
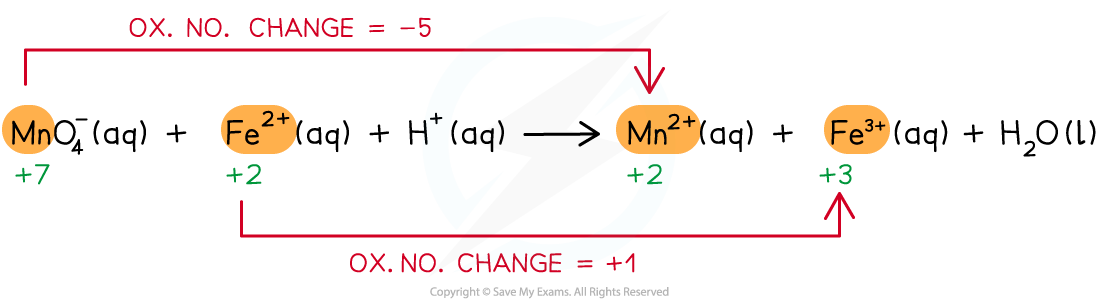
Step 3: Balance the oxidation number changes
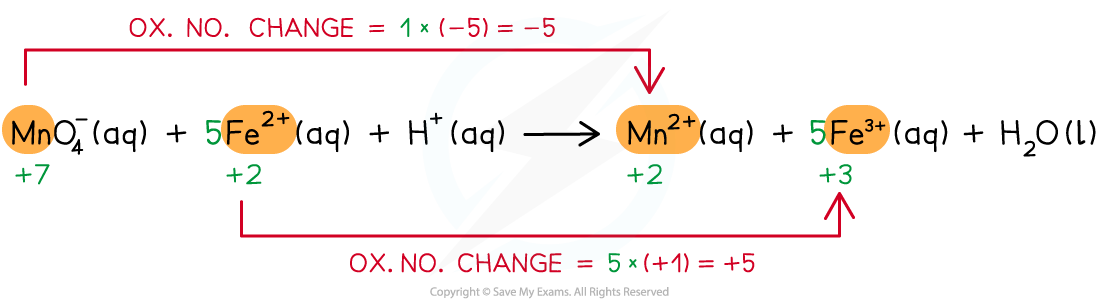
Step 4: Balance the charges
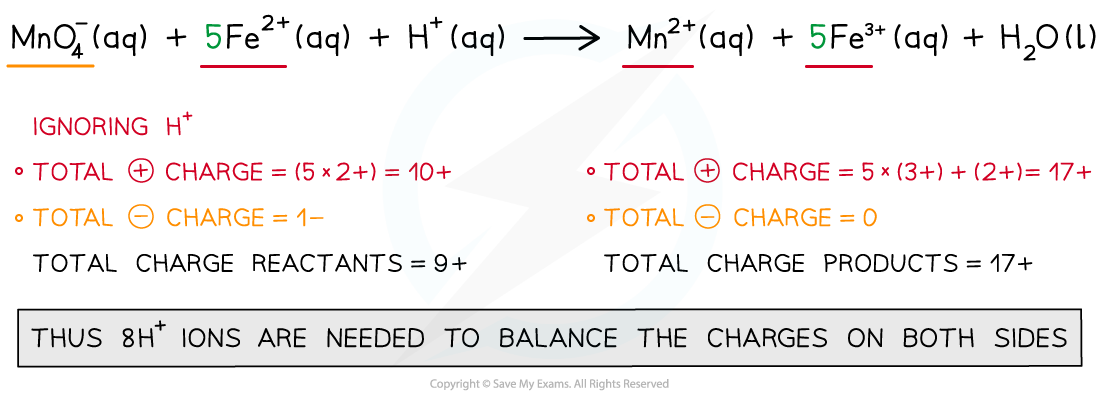
Step 5: Finally, balance the atoms

Metals & Non-metals
Metals
- Metals, in general, will form positive ions by losing electrons
- Therefore, they are oxidised and the oxidation number increases
- Example 1:
- When sodium reacts with water, sodium hydroxide and hydrogen gas is formed
2Na (s) + H2O (l) → 2NaOH (aq) + H2 (g)
- The oxidation number of sodium changes from 0 to +1
- Example 2:
- When magnesium reacts with hydrochloric acid, magnesium chloride and hydrogen gas is formed
Mg (s) + 2HCl (l) → MgCl2 (aq) + H2 (g)
- The oxidation number of magnesium changed from 0 to +2
Non-metals
- Non-metals, in general, will form negative ions by gaining electrons
- Therefore, they are reduced and the oxidation number decreases
- Example:
- When sodium reacts with oxygen, sodium oxide is formed
4Na (s) + O2 (g) → Na2O (s)
- The oxidation number of oxygen changes from 0 to -2
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









