- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记2.1.3 Redox & Disproportionation
Disproportionation
Disproportionation reactions
- A disproportionation reaction is a reaction in which the same species is simultaneously oxidised and reduced
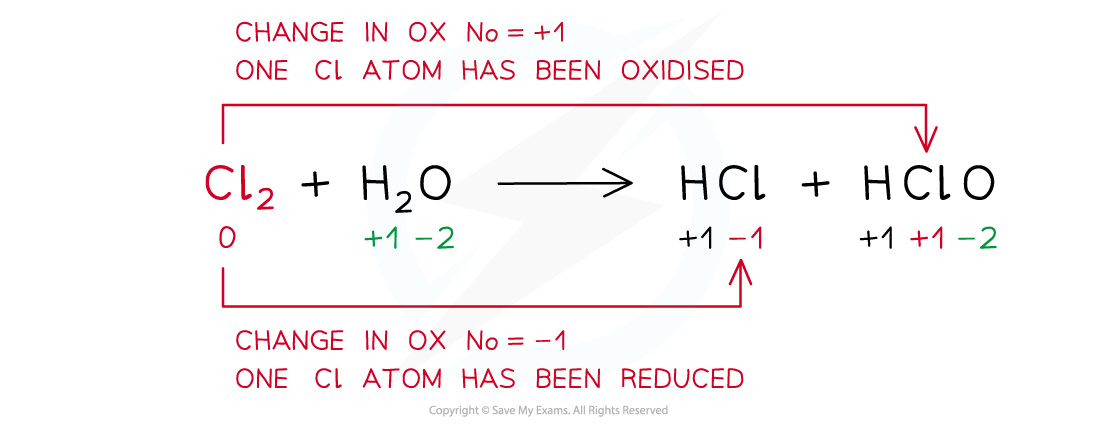
Example of a disproportion reaction in which the same species (chlorine in this case) has been both oxidised and reduced
Using Oxidation Numbers
Worked Example
Balancing disproportionation reactions
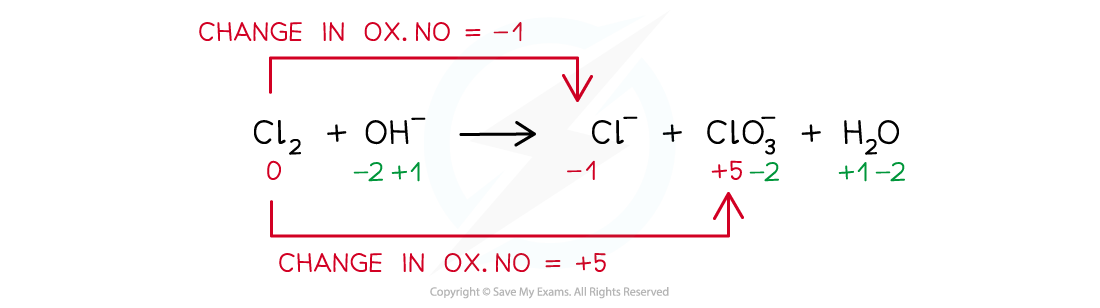
Balance the disproportionation reaction which takes place when chlorine is added to hot concentrated aqueous sodium hydroxide. The products are Cl- and ClO3- ions and water
Answer
Step 1: Write the unbalanced equation and identify the atoms that change in oxidation number:

Step 2: Deduce the oxidation number changes:
Step 3: Balance the oxidation number changes:
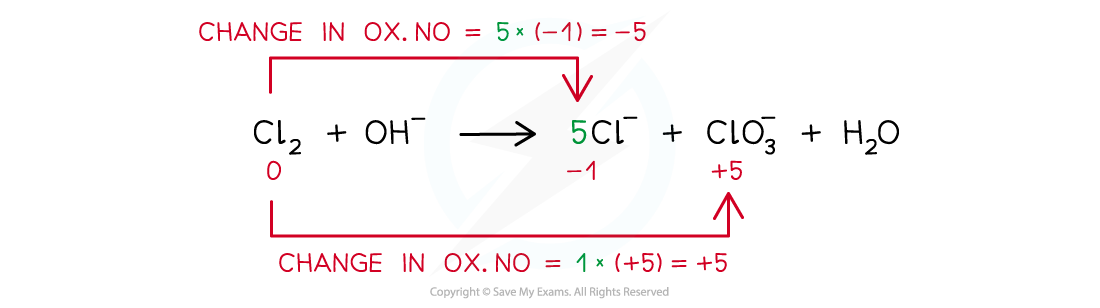
Step 4: Balance the charges

Step 5: Balance the atoms

Worked Example
Oxidation and reduction
In each of the following equations, state which reactant has been oxidised and which has been reduced.
- Na++ Cl- → NaCl
- Mg + Fe2+ → Mg2+ + Fe
- CO + Ag2O → 2Ag + CO2
Answer 1:
-
- Oxidised: Cl- as the oxidation state has increased by 1
- Reduced: Na+ as the oxidation state has decreased by 1
Answer 2:
-
- Oxidised: Mg as the oxidation state has increased by 2
- Reduced: Fe2+ as the oxidation state has decreased by 2
Answer 3:
-
- Oxidised: C as it has gained oxygen
- Reduced: Ag as it has lost oxygen
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









