- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记1.10.4 Deducing Kc Expressions
Deducing Kc Expressions
Equilibrium expression & constant
- The equilibrium expression links the equilibrium constant, Kc, to the concentrations of reactants and products at equilibrium taking the stoichiometry of the equation into account
- So, for a given reaction:
aA + bB ⇌ cC + dD
- Kc is defined as follows:
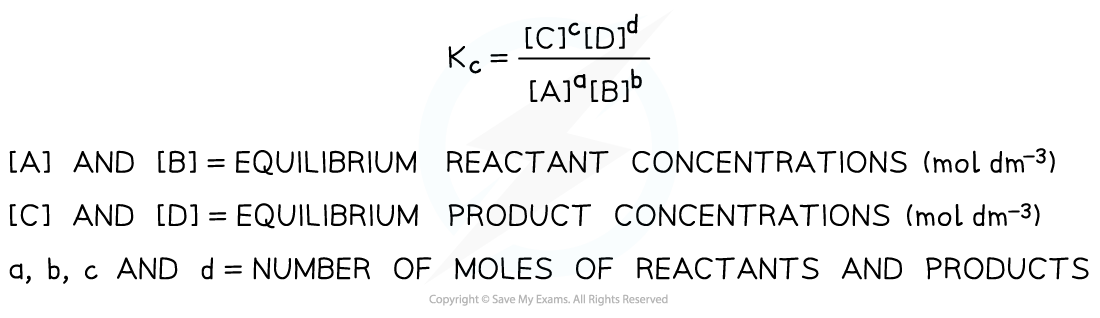
Equilibrium expression linking the equilibrium concentration of reactants and products at equilibrium
- The Kc of a reaction is specific and only changes if the temperature of the reaction changes
Homogeneous systems and Kc
- A homogeneous system is where all of the reactants and products are in the same physical state, e.g.
CH3COOH (l) + C2H5OH (l) ⇌ CH3COOC2H5 (l) + H2O (l)
- For this reaction, all of the reactants and products are in the same, liquid state / phase and will, therefore, all feature in the Kc expression
- Kc =
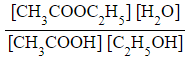
- Kc =
Heterogeneous systems and Kc
- A heterogeneous system is where not all of the reactants and products are in the same physical state, e.g.
CaCO3 (s) ⇌ CaO (s) + CO2 (g)
- Solids are ignored in equilibrium expressions
- This leads to a Kc expression of Kc = [CO2]
Exam Tip
For Kc expressions, it is important that you use square brackets as sometimes examiners are instructed to be strict about the appearance of brackets in expressions
Square brackets implies concentration
Worked Example
Deducing equilibrium expressions
Deduce the equilibrium expression for the following reactions:
- Ag+ (aq) + Fe2+ (aq) ⇌ Ag (s) + Fe3+ (aq)
- N2 (g) + 3H2 (g) ⇌ 2NH3 (g)
- 2SO2 (g) + O2 (g) ⇌ 2SO3 (g)
Answer 1:
-
- Kc =
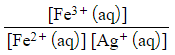
- [Ag (s)] is not included in the equilibrium expression as it is a solid
- Kc =
Answer 2:
-
- Kc =
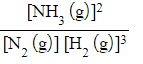
- Kc =
Answer 3:
-
- Kc =
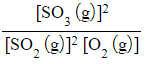
- Kc =
转载自savemyexams


最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








