- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记1.10.2 Le Chatelier's Principle
Le Chatelier's Principle
Position of the equilibrium
- The position of the equilibrium refers to the relative amounts of products and reactants in an equilibrium mixture
- When the position of equilibrium shifts to the left, it means the concentration of reactants increases
- When the position of equilibrium shifts to the right, it means the concentration of products increases
Le Chatelier’s principle
- Le Chatelier’s principle says that if a change is made to a system in dynamic equilibrium, the position of the equilibrium moves to counteract this change
- The principle is used to predict changes to the position of equilibrium when there are changes in temperature, pressure or concentration
Effects of concentration
How the equilibrium shifts with concentration changes

Worked Example
Changes in equilibrium position
Using the reaction below:
CH3COOH (I) + C2H5OH (I) ⇌ CH3COOC2H5 (I) + H2O (I)
Explain what happens to the position of equilibrium when:
1. More CH3COOC2H5 is added
2. Some C2H5OH is removed
Using the reaction below:
Ce4+ (aq) + Fe2+ (aq) ⇌ Ce3+ (aq) + Fe3+ (aq)
Explain what happens to the position of equilibrium when
3. Water is added to the equilibrium mixture
Answer 1:
-
- The position of the equilibrium moves to the left and more ethanoic acid and ethanol are formed
- The reaction moves in this direction to oppose the effect of added ethyl ethanoate, so the ethyl ethanoate decreases in concentration
Answer 2:
-
- The position of the equilibrium moves to the left and more ethanoic acid and ethanol are formed
- The reaction moves in this direction to oppose the removal of ethanol so more ethanol (and ethanoic acid) are formed from ethyl ethanoate and water
Answer 3:
-
- There is no effect as the water dilutes all the ions equally so there is no change in the ratio of reactants to products
Effects of pressure
- Changes in pressure only affect reactions where the reactants or products are gases
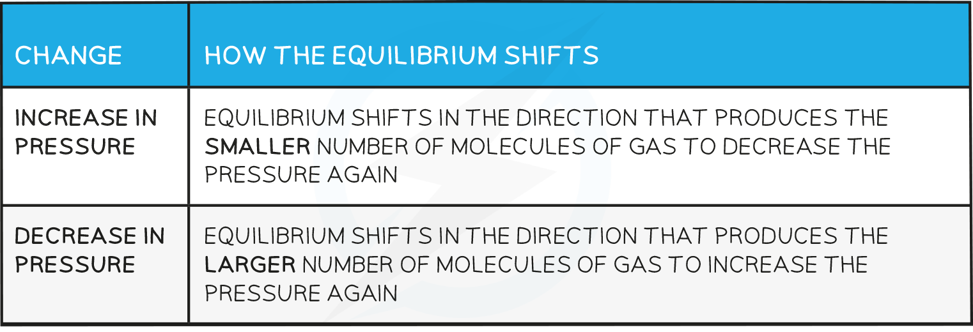
How the equilibrium shifts with pressure changes
Worked Example
Changes in pressure
Predict the effect of increasing the pressure on the following reactions:
1. N2O4 (g) ⇌ 2NO2 (g)
2. CaCO3 (s) ⇌ CaO (s) + CO2 (g)
Predict the effect of decreasing the pressure on the following reaction:
3. 2NO2 (g) ⇌ 2NO (g) + O2 (g)
Answer 1:
-
- The equilibrium shifts to the left as there are fewer gas molecules on the left
- This causes a decrease in pressure
Answer 2:
-
- The equilibrium shifts to the left as there are no gas molecules on the left but there is CO2 on the right
- This causes a decrease in pressure
Answer 3:
-
- The equilibrium shifts to the right as there is a greater number of gas molecules on the right
- This causes an increase in pressure
Effects of temperature
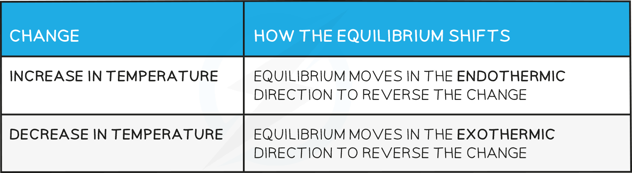
How the equilibrium shifts with temperature changes
Worked Example
Changes in temperature
Using the reaction below:
H2 (g) + CO2 (g) ⇌ H2O (g) + CO (g) ΔH = +41.2 kJ mol-1
1. Predict the effect of increasing the temperature on this reaction
Using the reaction below:
Ag2CO3 (s) ⇌ Ag2O (s) + CO2 (g)
2. Increasing the temperature increases the amount of CO2(g) at constant pressure. Is this reaction exothermic or endothermic?
Explain your answer
Answer 1:
-
- The reaction will absorb the excess energy and since the forward reaction is endothermic, the equilibrium will shift to the right
Answer 2:
-
- The reaction will absorb the excess energy and since this causes a shift of the equilibrium towards the right (as more CO2(g) is formed) this means that the reaction is endothermic
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









