- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记1.6.5 Amount of Substance Calculations
Amount of Substance Calculations
- As previously discussed, the amount of substance can be defined as the number of particles in a substance, n, measured in moles (often abbreviated to mol)
- In reality, amount of substance is used as a blanket term to cover most chemical calculations, especially those that involve moles
- The two main calculations for amount of substance are:
Moles, n = 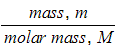
Moles, n = concentration x volume
- Other common calculations for amount of substance include:
Moles, n = 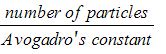
Moles, n =![]() from the ideal gas equation (PV = nRT)
from the ideal gas equation (PV = nRT)
Reacting Mass Calculations
- Chemical equations can be used to calculate the moles or masses of reactants and products
- To do this, information given in the question is used to find the amount in moles of the substances being considered
- Then, the ratio between the substances is identified using the balanced chemical equation
- Once the moles have been determined they can then be converted into grams using the relative atomic or relative formula masses
Worked Example
Example 1
Calculate the mass of magnesium oxide that can be made by completely burning 6.0 g of magnesium in oxygen in the following reaction:
2Mg (s) + O2 (g) ⟶ 2 MgO (s)
Answer

Worked Example
Example 2
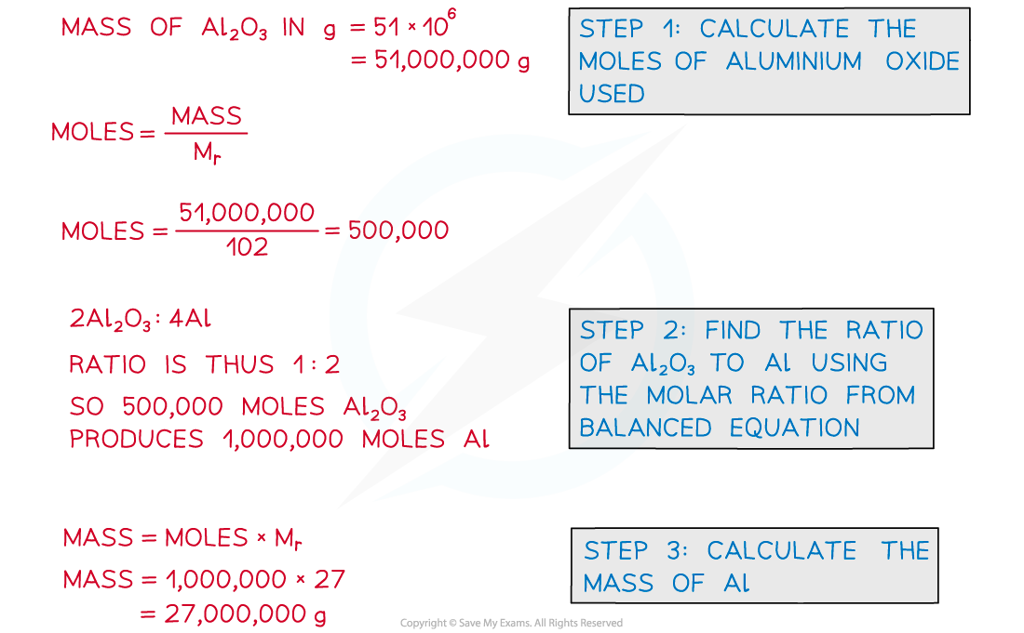
Calculate the mass of aluminium, in tonnes, that can be produced from 51 tonnes of aluminium oxide. The equation for the reaction is:
2Al2O3 ⟶ 4Al + 3O2
Answer

Exam Tip
As long as you are consistent it doesn't matter whether you work in grams or tonnes or any other mass unit as the reacting masses will always be in proportion to the balanced equation.
Balancing Equations using Reacting Masses
- If the masses of reactants and products of a reaction are known then we can use them to write a balanced equation for that reaction
- This is done by converting the masses to moles and simplifying to find the molar ratios
Worked Example
Example 3
A student reacts 1.2 g of carbon with 16.2 g of zinc oxide. The resulting products are 4.4 g of carbon dioxide and 13 g of zinc. Determine the balanced equation for the reaction.
Answer


Exam Tip
These questions look hard but they are actually quite easy to do, as long as you follow the steps and organise your work neatly.
Remember the molar ratio of a balanced equation gives you the ratio of the amounts of each substance in the reaction.
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








