- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记1.2.3 Orbitals
Orbital Shapes
Orbitals
- Subshells contain one or more atomic orbitals
- Orbitals exist at specific energy levels and electrons can only be found at these specific levels, not in between them
- Each atomic orbital can be occupied by a maximum of two electrons
- This means that the number of orbitals in each subshell is as follows:
- s : one orbital (1 x 2 = total of 2 electrons)
- p : three orbitals ( 3 x 2 = total of 6 electrons)
- d : five orbitals (5 x 2 = total of 10 electrons)
- f : seven orbitals (7 x 2 = total of 14 electrons)
- The orbitals have specific 3-D shapes
s orbital shape
- The s orbitals are spherical in shape
- The size of the s orbitals increases with increasing shell number
- E.g. the s orbital of the third quantum shell (n = 3) is bigger than the s orbital of the first quantum shell (n = 1)
p orbital shape
- The p orbitals have a dumbbell shape
- Every shell has three p orbitals except for the first one (n = 1)
- The p orbitals occupy the x, y and z axes and point at right angles to each other, so are oriented perpendicular to one another
- The lobes of the p orbitals become larger and longer with increasing shell number
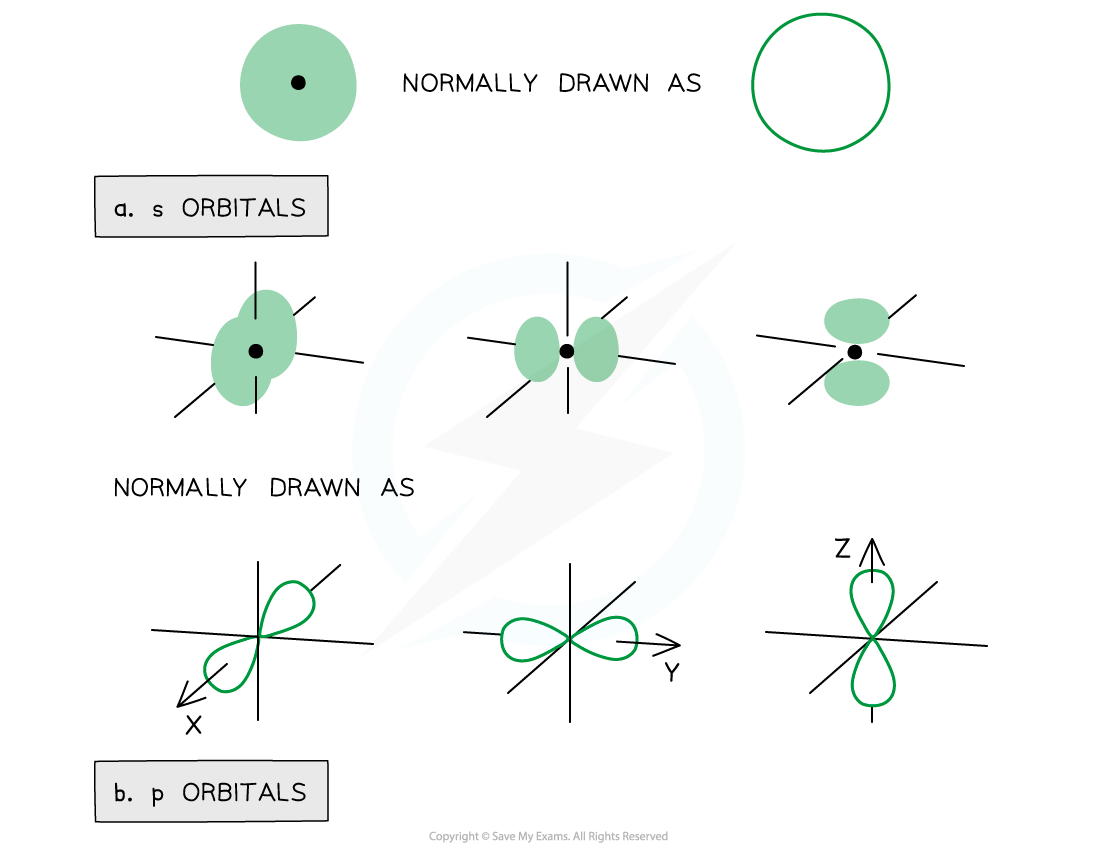
Representation of orbitals (the dot represents the nucleus of the atom) showing spherical s orbitals (a), p orbitals containing ‘lobes’ along the x, y and z axis
- Note that the shape of the d orbitals is not required
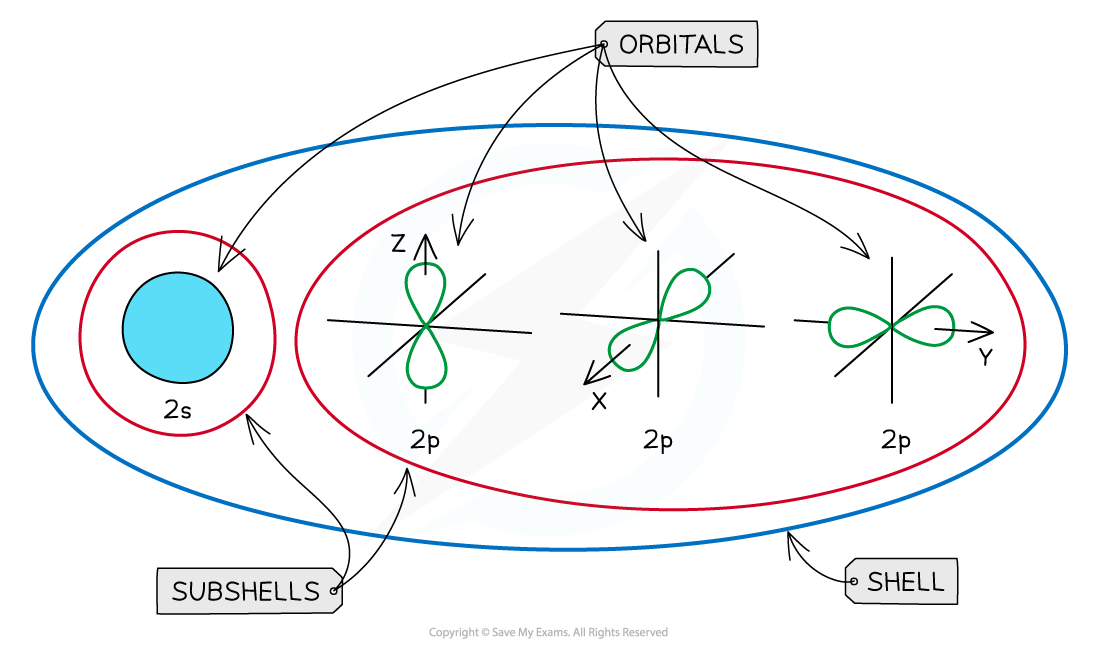
An overview of the shells, subshells and orbitals in an atom
Filling Orbitals
- Electrons can be imagined as small spinning charges which rotate around their own axis in either a clockwise or anticlockwise direction
- The spin of the electron is represented by its direction
- The spin creates a tiny magnetic field with N-S pole pointing up or down
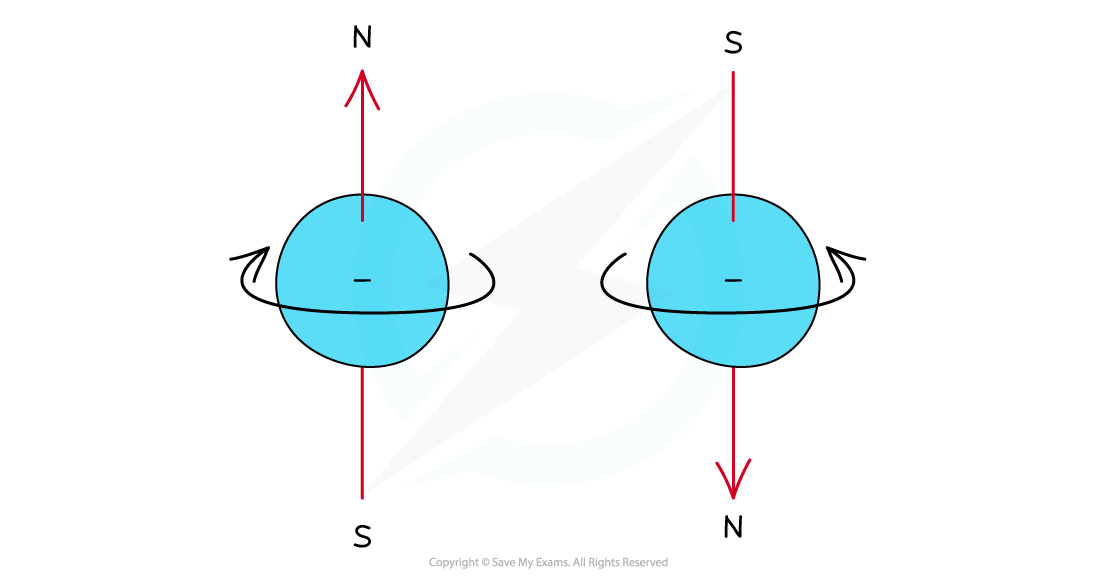
Electrons can spin either in a clockwise or anticlockwise direction around their own axis
- Electrons with the same spin repel each other which is also called spin-pair repulsion
- Therefore, electrons will occupy separate orbitals in the same subshell first to minimise this repulsion and have their spin in the same direction
- They will then pair up, with a second electron being added to the first p orbital, with its spin in the opposite direction
- This is known as Hund's Rule
- E.g. if there are three electrons in a p subshell, one electron will go into each px, py and pz orbital

Electron configuration: three electrons in a p subshell
- The principal quantum number indicates the energy level of a particular shell but also indicates the energy of the electrons in that shell
- A 2p electron is in the second shell and therefore has an energy corresponding to n = 2
- Even though there is repulsion between negatively charged electrons, they occupy the same region of space in orbitals
- An orbital can only hold two electrons and they must have opposite spin - the is known as the Pauli Exclusion Principle
- This is because the energy required to jump to a higher empty orbital is greater than the inter-electron repulsion
- For this reason, they pair up and occupy the lower energy levels first
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









