- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Physics复习笔记23.1.3 Mass Defect & Binding Energy
Mass Defect & Binding Energy
- Experiments into nuclear structure have found that the total mass of a nucleus is less than the sum of the masses of its constituent nucleons
- This difference in mass is known as the mass defect
- Mass defect is defined as:
The difference between an atom's mass and the sum of the masses of its protons and neutrons
- The mass defect Δm of a nucleus can be calculated using:
Δm = Zmp + (A – Z)mn – mtotal
- Where:
- Z = proton number
- A = nucleon number
- mp = mass of a proton (kg)
- mn = mass of a neutron (kg)
- mtotal = measured mass of the nucleus (kg)
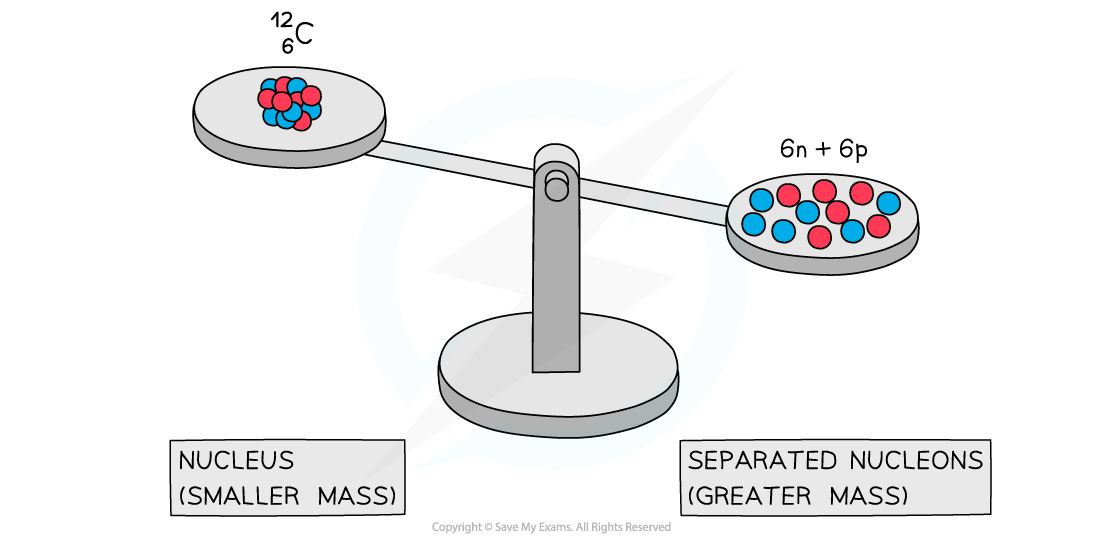
A system of separated nucleons has a greater mass than a system of bound nucleons
- Due to the equivalence of mass and energy, this decrease in mass implies that energy is released in the process
- Since nuclei are made up of neutrons and protons, there are forces of repulsion between the positive protons
- Therefore, it takes energy, ie. the binding energy, to hold nucleons together as a nucleus
- Binding energy is defined as:
The energy required to break a nucleus into its constituent protons and neutrons
- Energy and mass are proportional, so, the total energy of a nucleus is less than the sum of the energies of its constituent nucleons
- The formation of a nucleus from a system of isolated protons and neutrons is therefore an exothermic reaction - meaning that it releases energy
- This can be calculated using the equation:
E = Δmc2
Exam Tip
Avoid describing the binding energy as the energy stored in the nucleus – this is not correct – it is energy that must be put into the nucleus to pull it apart.
Binding Energy per Nucleon
- In order to compare nuclear stability, it is more useful to look at the binding energy per nucleon
- The binding energy per nucleon is defined as:
The binding energy of a nucleus divided by the number of nucleons in the nucleus
- A higher binding energy per nucleon indicates a higher stability
- In other words, it requires more energy to pull the nucleus apart
- Iron (A = 56) has the highest binding energy per nucleon, which makes it the most stable of all the elements
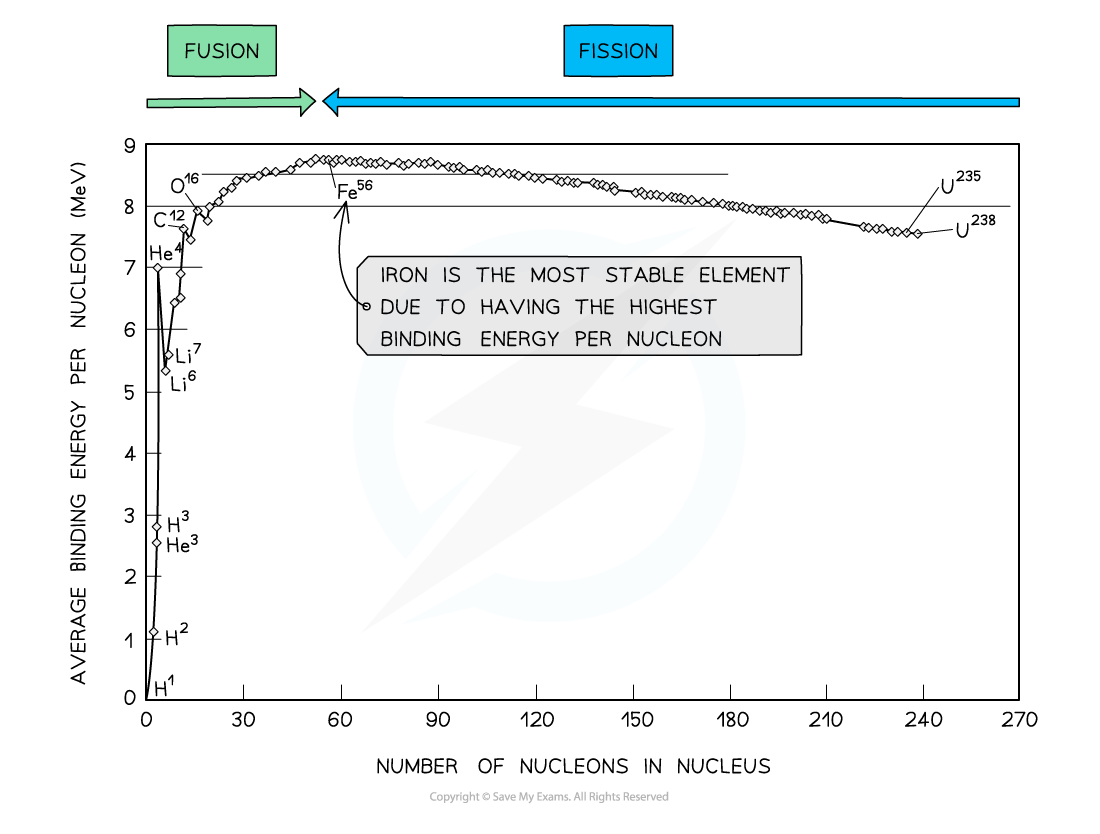
By plotting a graph of binding energy per nucleon against nucleon number, the stability of elements can be inferred
Key Features of the Graph
- At low values of A:
- Nuclei tend to have a lower binding energy per nucleon, hence, they are generally less stable
- This means the lightest elements have weaker electrostatic forces and are the most likely to undergo fusion
- Helium (4He), carbon (12C) and oxygen (16O) do not fit the trend
- Helium-4 is a particularly stable nucleus hence it has a high binding energy per nucleon
- Carbon-12 and oxygen-16 can be considered to be three and four helium nuclei, respectively, bound together
- At high values of A:
- The general binding energy per nucleon is high and gradually decreases with A
- This means the heaviest elements are the most unstable and likely to undergo fission
Worked Example

Step 1: Calculate the mass defect
Number of protons, Z = 26
Number of neutrons, A – Z = 56 – 26 = 30
Mass defect, Δm = Zmp + (A – Z)mn – mtotal
Δm = (26 × 1.673 × 10-27) + (30 × 1.675 × 10-27) – (9.288 × 10-26)
Δm = 8.680 × 10-28 kg
Step 2: Calculate the binding energy of the nucleus
Binding energy, E = Δmc2
E = (8.680 × 10-28) × (3.00 × 108)2 = 7.812 × 10-11 J
Step 3: Calculate the binding energy per nucleon
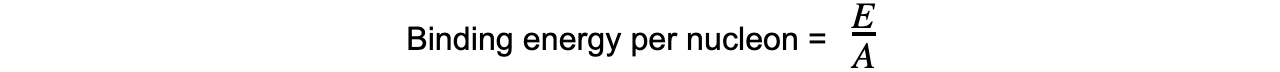
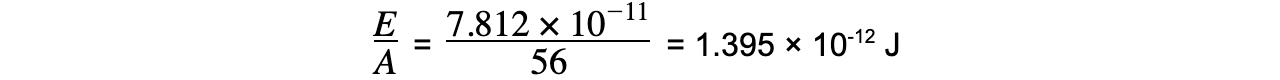 Step 4: Convert to MeV
Step 4: Convert to MeV
J → eV: divide by 1.6 × 10-19
eV → MeV: divide by 106
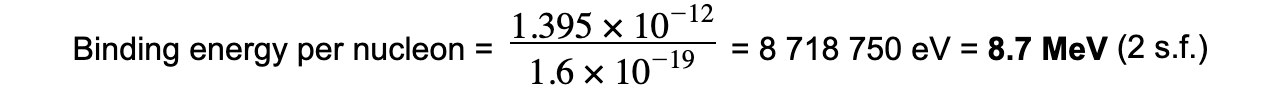
Exam Tip
Checklist on what to include (and what not to include) in an exam question asking you to draw a graph of binding energy per nucleon against nucleon number:
- You will be expected to draw the best fit curve AND a cross to show the anomaly that is helium
- Do not begin your curve at A = 0, this is not a nucleus!
- Make sure to correctly label both axes AND units for binding energy per nucleon
- You will be expected to include numbers on the axes, mainly at the peak to show the position of iron (56Fe)
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









