- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Physics复习笔记18.1.5 Electric Force Between Two Point Charges
Coulomb's Law
- All charged particles produce an electric field around it
- This field exerts a force on any other charged particle within range
- The electrostatic force between two charges is defined by Coulomb’s Law
- Recall that the charge of a uniform spherical conductor can be considered as a point charge at its centre
- Coulomb’s Law states that:
The electrostatic force between two point charges is proportional to the product of the charges and inversely proportional to the square of their separation
- The Coulomb equation is defined as:
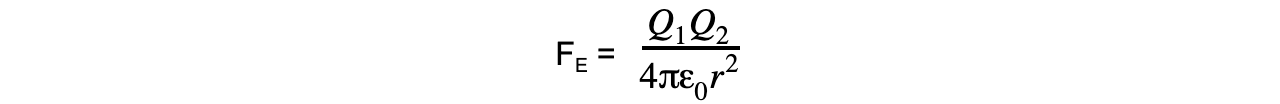

The electrostatic force between two charges is defined by Coulomb’s Law
- Where:
- FE = electrostatic force between two charges (N)
- Q1 and Q2 = two point charges (C)
- ε0 = permittivity of free space
- r = distance between the centre of the charges (m)
- The 1/r2 relation is called the inverse square law
- This means that when a charge is twice as far as away from another, the electrostatic force between them reduces by (½)2 = ¼
- If there is a positive and negative charge, then the electrostatic force is negative, this can be interpreted as an attractive force
- If the charges are the same, the electrostatic force is positive, this can be interpreted as a repulsive force
- Since uniformly charged spheres can be considered as point charges, Coulomb’s law can be applied to find the electrostatic force between them as long as the separation is taken from the centre of both spheres
Worked Example
An alpha particle is situated 2.0 mm away from a gold nucleus in a vacuum. Assuming them to be point charges, calculate the magnitude of the electrostatic force acting on each of the charges.Atomic number of helium = 2Atomic number of gold = 79Charge of an electron = 1.60 × 10-19 C
Step 1: Write down the known quantities
-
- Distance, r = 2.0 mm =2.0 × 10-3 m
The charge of one proton = +1.60 × 10-19 C
An alpha particle (helium nucleus) has 2 protons
-
- Charge of alpha particle, Q1 = 2 × 1.60 × 10-19 = +3.2 × 10-19 C
The gold nucleus has 79 protons
-
- Charge of gold nucleus, Q2 = 79 × 1.60 × 10-19 = +1.264 × 10-17 C
Step 2: The electrostatic force between two point charges is given by Coulomb’s Law
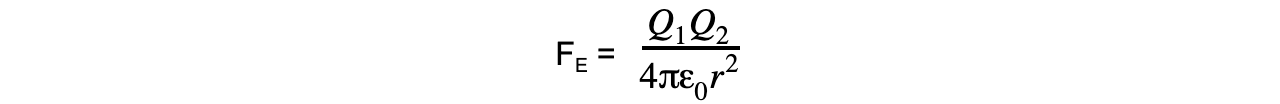
Step 3: Substitute values into Coulomb's Law
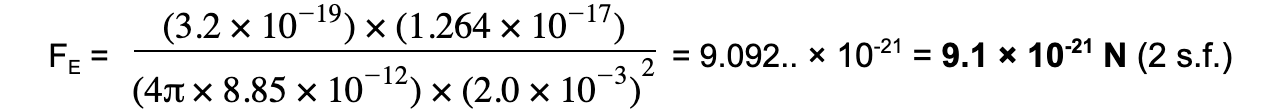
转载自savemyexams


最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








