- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Physics复习笔记15.2.1 Kinetic Theory of Gases
Assumptions of the Kinetic Theory of Gases
- Gases consist of atoms or molecules randomly moving around at high speeds
- The kinetic theory of gases models the thermodynamic behaviour of gases by linking the microscopic properties of particles (mass and speed) to macroscopic properties of particles (pressure and volume)
The theory is based on a set of the following assumptions:
-
- Molecules of gas behave as identical, hard, perfectly elastic spheres
- The volume of the molecules is negligible compared to the volume of the container
- The time of a collision is negligible compared to the time between collisions
- There are no forces of attraction or repulsion between the molecules
- The molecules are in continuous random motion
- The number of molecules of gas in a container is very large, therefore the average behaviour (eg. speed) is usually considered
Exam Tip
Make sure to memorise all the assumptions for your exams, as it is a common exam question to be asked to recall them.
Root-Mean-Square Speed
- The pressure of an ideal gas equation includes the mean square speed of the particles:
<c2>
- Where
- c = average speed of the gas particles
- <c2> has the units m2 s-2
- Since particles travel in all directions in 3D space and velocity is a vector, some particles will have a negative direction and others a positive direction
- When there are a large number of particles, the total positive and negative velocity values will cancel out, giving a net zero value overall
- In order to find the pressure of the gas, the velocities must be squared
- This is a more useful method, since a negative or positive number squared is always positive
- To calculate the average speed of the particles in a gas, take the square root of the mean square speed:

- cr.m.s is known as the root-mean-square speed and still has the units of m s-1
- The mean square speed is not the same as the mean speed
Worked Example
An ideal gas has a density of 4.5 kg m-3 at a pressure of 9.3 × 105 Pa and a temperature of 504 K.Determine the root-mean-square (r.m.s.) speed of the gas atoms at 504 K.
Step 1: Write out the equation for the pressure of an ideal gas with density
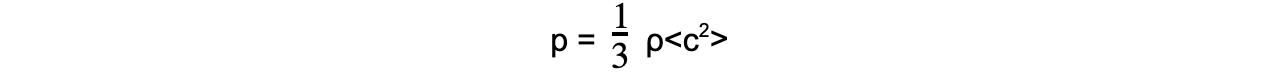
Step 2: Rearrange for mean square speed
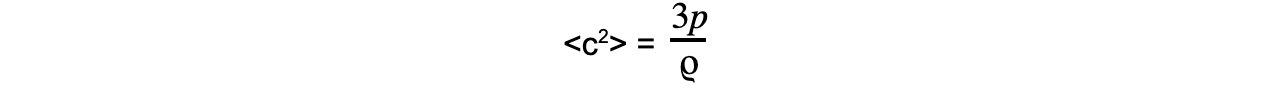
Step 3: Substitute in values
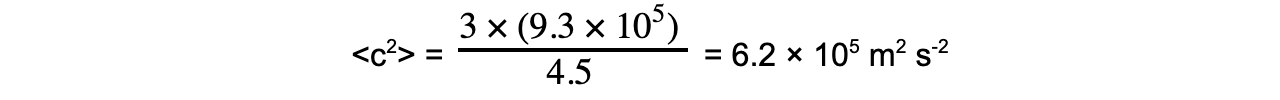
Step 4: To find the r.m.s value, take the square root of the mean square speed
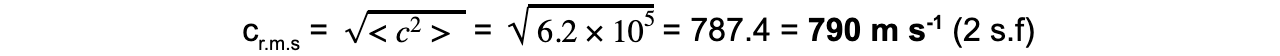
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









