- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Physics复习笔记15.1.3 Ideal Gas Equation
Ideal Gas Equation
- The equation of state for an ideal gas (or the ideal gas equation) can be expressed as:
pV = nRT
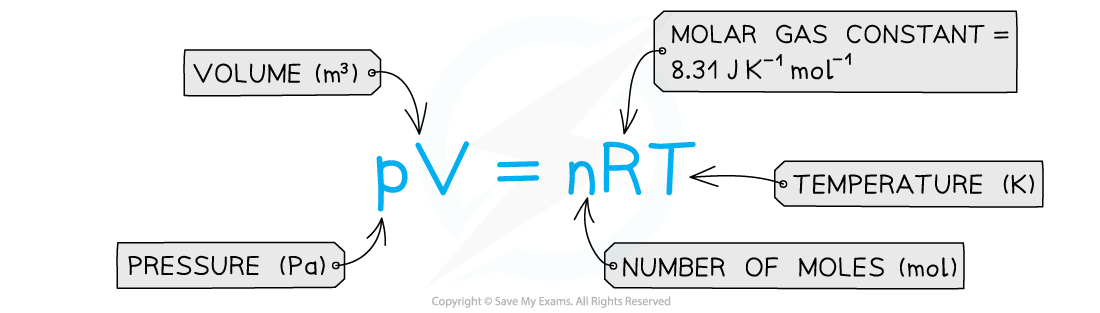
- The ideal gas equation can also be written in the form:
pV = NkT
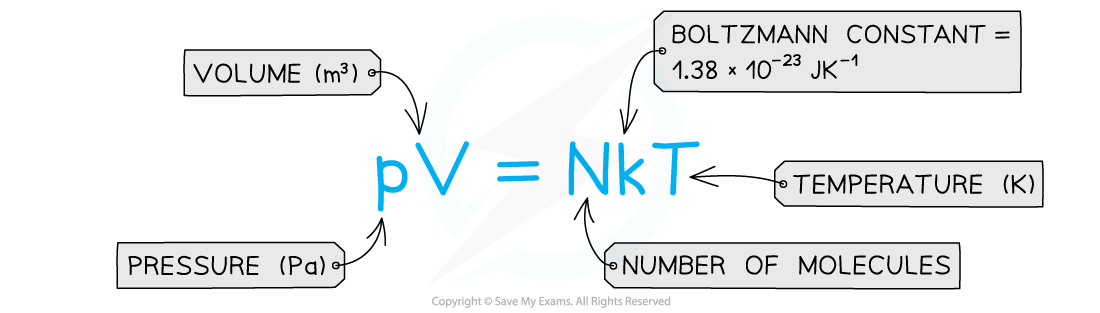
- An ideal gas is therefore defined as:
A gas which obeys the equation of state pV = nRT at all pressures, volumes and temperatures
Worked Example
A storage cylinder of an ideal gas has a volume of 8.3 × 103 cm3. The gas is at a temperature of 15oC and a pressure of 4.5 × 107 Pa. Calculate the amount of gas in the cylinder, in moles.
Step 1: Write down the ideal gas equation
Since the number of moles (n) is required, use the equation:
pV = nRT
Step 2: Rearrange for the number of moles n
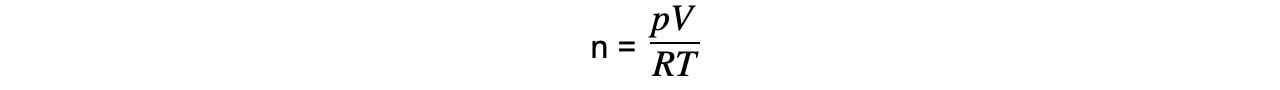
Step 3: Substitute in values
V = 8.3 × 103 cm3 = 8.3 × 103 × 10-6 = 8.3 × 10-3 m3
T = 15 oC + 273.15 = 288.15 K
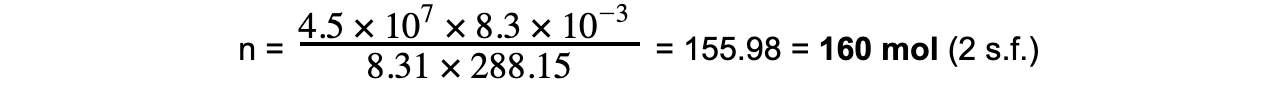
Exam Tip
Don’t worry about remembering the values of R and k, they will both be given in the equation sheet in your exam.
The Boltzmann Constant
- The Boltzmann constant k is used in the ideal gas equation and is defined by the equation:
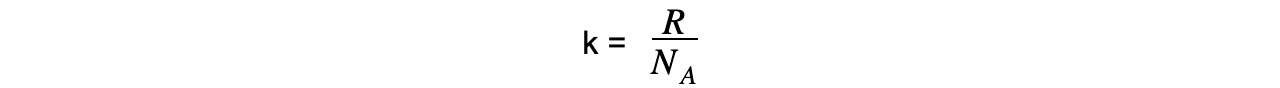
- Where:
- R = molar gas constant
- NA = Avogadro’s constant
- Boltzmann’s constant therefore has a value of
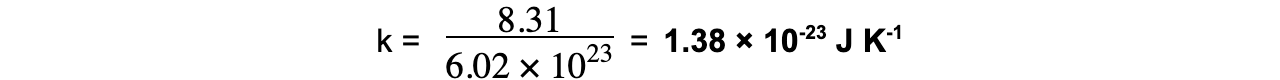
- The Boltzmann constant relates the properties of microscopic particles (e.g. kinetic energy of gas molecules) to their macroscopic properties (e.g. temperature)
- This is why the units are J K-1
- Its value is very small because the increase in kinetic energy of a molecule is very small for every incremental increase in temperature
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








