- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Physics复习笔记15.1.2 Ideal Gases
Ideal Gases
- An ideal gas is one which obeys the relation:
pV ∝ T
- Where:
- p = pressure of the gas (Pa)
- V = volume of the gas (m3)
- T = thermodynamic temperature (K)
- The molecules in a gas move around randomly at high speeds, colliding with surfaces and exerting pressure upon them
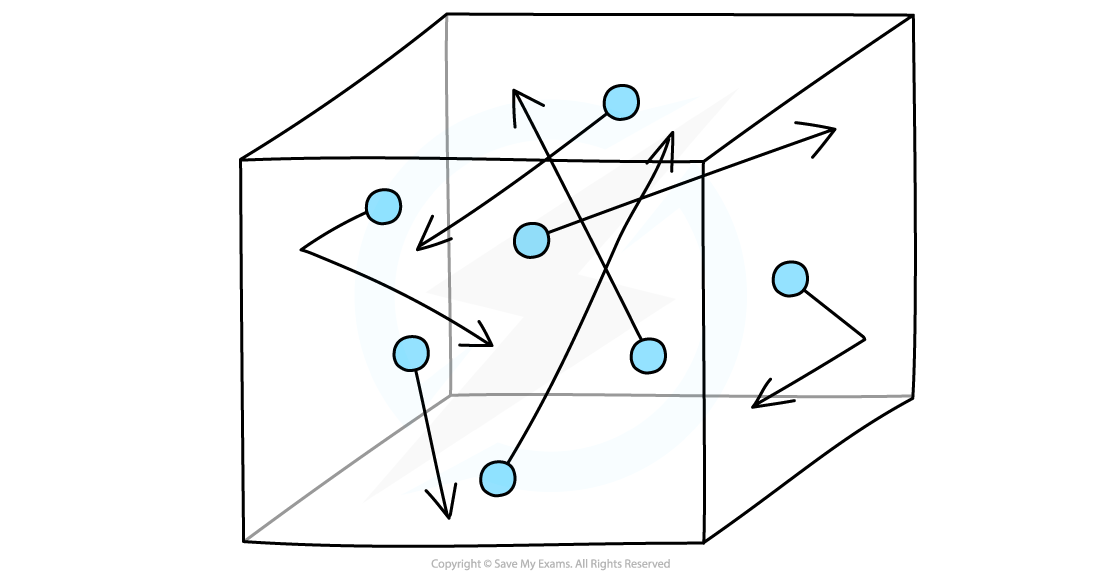
Gas molecules move about randomly at high speeds
- Imagine molecules of gas free to move around in a box
- The temperature of a gas is related to the average speed of the molecules:
- The hotter the gas, the faster the molecules move
- Hence the molecules collide with the surface of the walls more frequently
- Since force is the rate of change of momentum:
- Each collision applies a force across the surface area of the walls
- The faster the molecules hit the walls, the greater the force on them
- Since pressure is the force per unit area
- Higher temperature leads to higher pressure
- If the volume V of the box decreases, and the temperature T stays constant:
- There will be a smaller surface area of the walls and hence more collisions
- This also creates more pressure
- Since this equates to a greater force per unit area, pressure in an ideal gas is therefore defined by:
The frequency of collisions of the gas molecules per unit area of a container
Boyle’s Law
- If the temperature T is constant, then Boyle’s Law is given by:
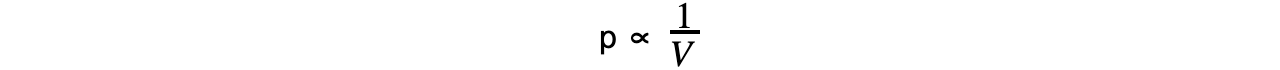
- This leads to the relationship between the pressure and volume for a fixed mass of gas at constant temperature:
P1V1 = P2V2
Charles's Law
- If the pressure P is constant, then Charles’s law is given by:
V ∝ T
- This leads to the relationship between the volume and thermodynamic temperature for a fixed mass of gas at constant pressure:
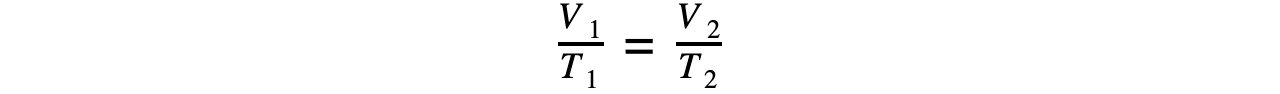
Pressure Law
- If the volume V is constant, the the Pressure law is given by:
P ∝ T
- This leads to the relationship between the pressure and thermodynamic temperature for a fixed mass of gas at constant volume:
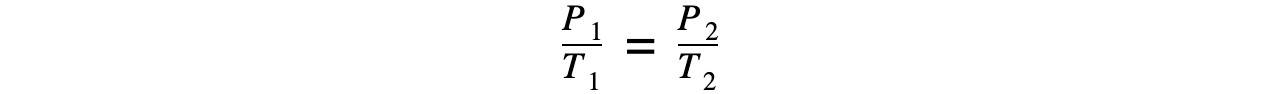
Worked Example
An ideal gas is in a container of volume 4.5 × 10−3 m3. The gas is at a temperature of 30°C and a pressure of 6.2 × 105 Pa.
Calculate the pressure of the ideal gas in the same container when it is heated to 40 °C.
Step 1: State the known values
-
- Volume, V = 4.5 × 10−3 m3
- Initial pressure, P1 = 6.2 × 105 Pa
- Initial temperature, T1 = 30°C = 303 K
- Initial temperature, T2 = 40°C = 313 K
Step 2: Since volume is constant, state the pressure law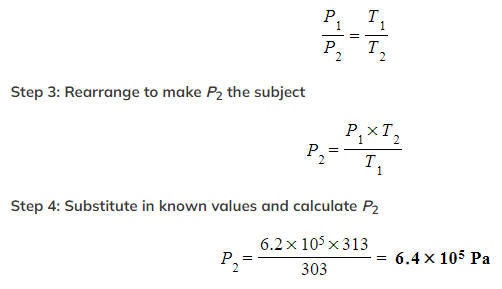
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









