- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Physics复习笔记15.1.1 The Mole
Amount of Substance
- In thermodynamics, the amount of substance is measured in the SI unit ‘mole’
- This has the symbol mol
- The mole is a unit of substance, not a unit of mass
- The mole is defined as:
The SI base unit of an ‘amount of substance’. It is the amount containing as many particles (e.g. atoms or molecules) as there are atoms in 12 g of carbon-12
- The mole is an important unit in thermodynamics
- If we consider the number of moles of two different gases under the same conditions, their physical properties are the same
The Avogadro Constant
- In AS Physics, the atomic mass unit (u) was introduced as approximately the mass of a proton or neutron = 1.66 × 10-27 kg
- This means that an atom or molecule has a mass approximately equal to the number of protons and neutrons it contains
- A carbon-12 atom has a mass of:
12 u = 12 × 1.66 × 10-27 = 1.99 × 10-26 kg
- The exact number for a mole is defined as the number of molecules in exactly 12 g of carbon:
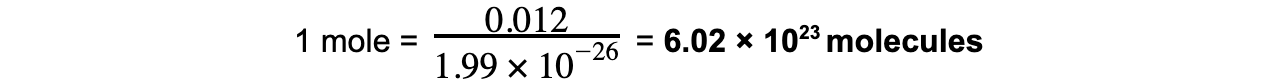
- Avogadro’s constant (NA) is defined as:
The number of atoms of carbon-12 in 12 g of carbon-12; equal to 6.02 × 1023 mol-1
- For example, 1 mole of sodium (Na) contains 6.02 × 1023 atoms of sodium
- The number of atoms can be determined if the number of moles is known by multiplying by NA, for example:
2.0 mol of nitrogen contains: 2.0 × NA = 2.0 × 6.02 × 1023 = 1.20 × 1024 atoms
Mole and the Atomic Mass
- One mole of any element is equal to the relative atomic mass of that element in grams
- E.g. Helium has an atomic mass of 4 - this means 1 mole of helium has a mass of 4 g
- If the substance is a compound, add up the relative atomic masses, for example, water (H2O) is made up of
- 2 hydrogen atoms (each with atomic mass of 1) and 1 oxygen atom (atomic mass of 16)
- So, 1 mole of water would have a mass of (2 × 1) + 16 = 18 g
Molar Mass
- The molar mass of a substance is the mass, in grams, in one mole
- Its unit is g mol-1
- The number of moles from this can be calculated using the equation:
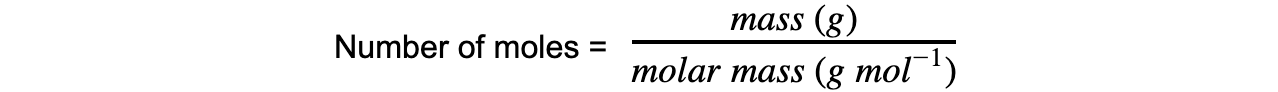
Worked Example
How many molecules are there in 6 g of magnesium-24?
Step 1: Calculate the mass of 1 mole of magnesium
One mole of any element is equal to the relative atomic mass of that
element in grams
1 mole = 24 g of magnesium
Step 2: Calculate the amount of moles in 6 g

Step 3: Convert the moles to number of molecules
1 mole = 6.02 × 1023 molecules
0.25 moles = 0.25 × 6.02 × 1023 = 1.51 × 1023 molecules
Exam Tip
If you want to find out more about the mole, check out the CIE A Level Chemistry revision notes!
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









