- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Physics复习笔记14.2.2 Specific Latent Heat Capacity
Defining Latent Heat Capacity
- Energy is required to change the state of substance
- Examples of changes of state are:
- Melting = solid to liquid
- Evaporation/vaporisation/boiling = liquid to gas
- Sublimation = solid to gas
- Freezing = liquid to solid
- Condensation = gas to liquid

The example of changes of state between solids, liquids and gases
- When a substance changes state, there is no temperature change
- The energy supplied to change the state is called the latent heat and is defined as:
The thermal energy required to change the state of 1 kg of mass of a substance without any change of temperature
- There are two types of latent heat:
- Specific latent heat of fusion (melting)
- Specific latent heat of vaporisation (boiling)
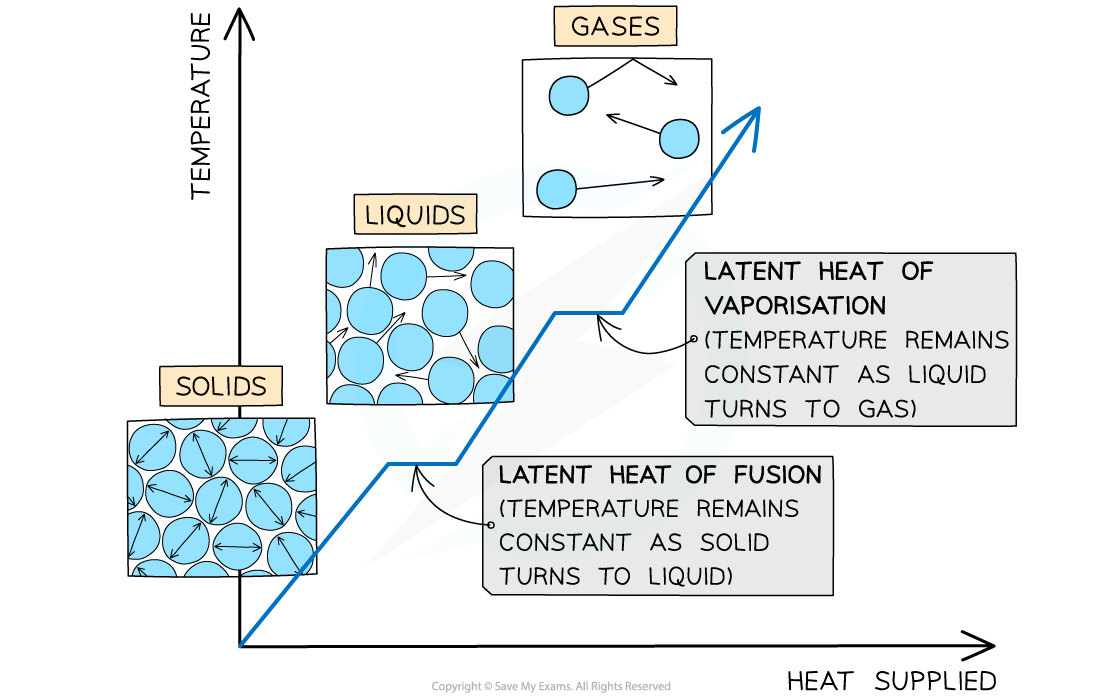
The changes of state with heat supplied against temperature. There is no change in temperature during changes of state
- The specific latent heat of fusion is defined as:
The thermal energy required to convert 1 kg of solid to liquid with no change in temperature
-
- This is used when melting a solid or freezing a liquid
- The specific latent heat of vaporisation is defined as:
The thermal energy required to convert 1 kg of liquid to gas with no change in temperature
-
- This is used when vaporising a liquid or condensing a gas
Calculating Specific Latent Heat
- The amount of energy Q required to melt or vaporise a mass of m with latent heat L is:
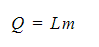 Where:
Where:
- Q = amount of thermal energy to change the state (J)
- L = latent heat of fusion or vaporisation (J kg-1)
- m = mass of the substance changing state (kg)
- The values of latent heat for water are:
- Specific latent heat of fusion = 330 kJ kg-1
- Specific latent heat of vaporisation = 2.26 MJ kg-1
- Therefore, evaporating 1 kg of water requires roughly seven times more energy than melting the same amount of ice to form water
- The reason for this is to do with intermolecular forces:
- When ice melts: energy is required to just increase the molecular separation until they can flow freely over each other
- When water boils: energy is required to completely separate the molecules until there are no longer forces of attraction between the molecules, hence this requires much more energy
Worked Example
The energy needed to boil a mass of 530 g of a liquid is 0.6 MJ. Calculate the specific latent heat of the liquid and state whether it is the latent heat of vaporisation or fusion.
Step 1: Write the thermal energy required to change state equation
![]()
Step 2: Rearrange for latent heat
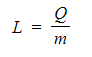 Step 3: Substitute in the values
Step 3: Substitute in the values
m = 530 g = 530 × 10-3 kg
Q = 0.6 MJ = 0.6 × 106 J
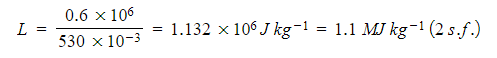 L is the latent heat of vaporisation because the change in state is from liquid to gas (boiling)
L is the latent heat of vaporisation because the change in state is from liquid to gas (boiling)
Exam Tip
Use these reminders to help you remember which type of latent heat is being referred to:
- Latent heat of fusion = imagine ‘fusing’ the liquid molecules together to become a solid
- Latent heat of vaporisation = “water vapour” is steam, so imagine vaporising the liquid molecules into a gas
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








