- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Physics复习笔记11.1.2 Nucleon & Proton Number
Nucleon & Proton Number
- The atomic symbol of an element is used to describe the constituents of the nuclei
- An example of this notation for Lithium is:
 Atomic symbol for Lithium
Atomic symbol for Lithium
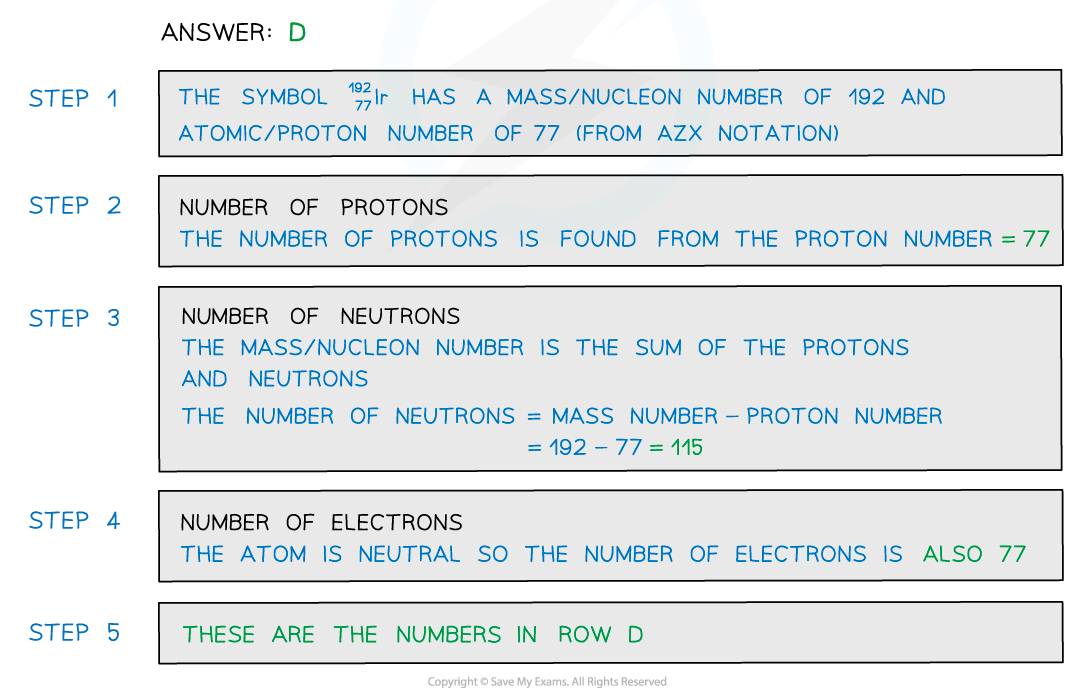
- When given an atomic symbol, you can figure out the number of protons, neutrons and electrons in the atom:
- Protons: The atomic number
- Electrons: Atoms are neutrals, so the number of negative electrons is equal to the number of positive protons. Therefore, this is also the atomic number
- Neutrons: Subtract the proton number from the mass number
- For the lithium atom, these numbers would be:
- Protons: 3
- Electrons: 3
- Neutrons: 7 − 3 = 4
- The term nucleon is the used to mean a particle in the nucleus – i.e. a proton or neutron
- The term nuclide is used to refer to a nucleus with a specific combination of protons and neutrons
Worked Example
Isotopes
- Although all atoms of the same element always have the same number of protons (and hence electrons), the number of neutrons can vary
- An isotope is an atom (of the same element) that has an equal number of protons but different number of neutrons
- The isotopes of hydrogen are deuterium and tritium:
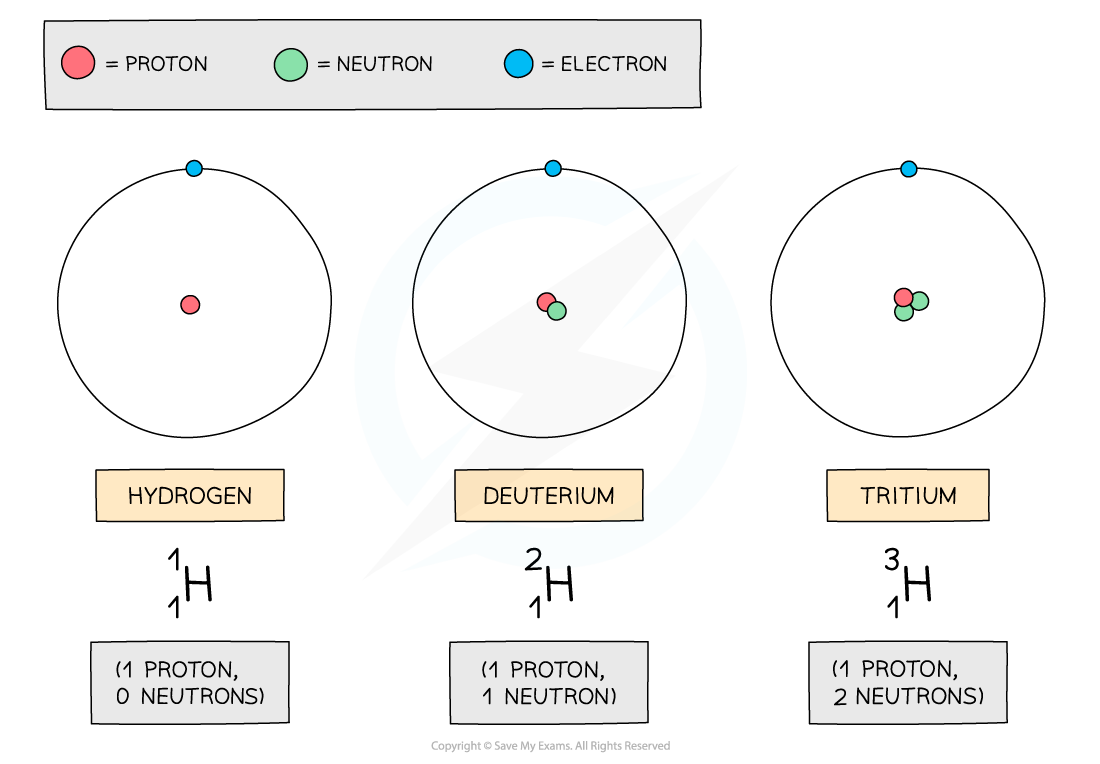
The three atoms shown above are all forms of hydrogen, but they each have different numbers of neutrons
- Remember, the neutron number of an atom is found by subtracting the proton number from the nucleon number
- Since nucleon number includes the number of neutrons, an isotope of an element will also have a different nucleon/mass number
- Since isotopes have an imbalance of neutrons and protons, they are unstable. This means they constantly decay and emit radiation to achieve a more stable form
- This can happen from anywhere between a few nanoseconds to 100,000 years
Worked Example
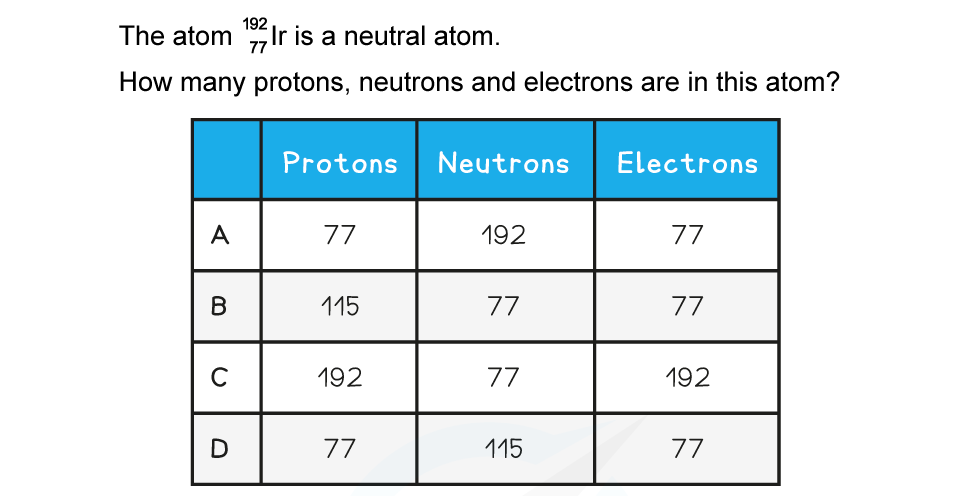
One of the rows in the table shows a pair of nuclei that are isotopes of one another.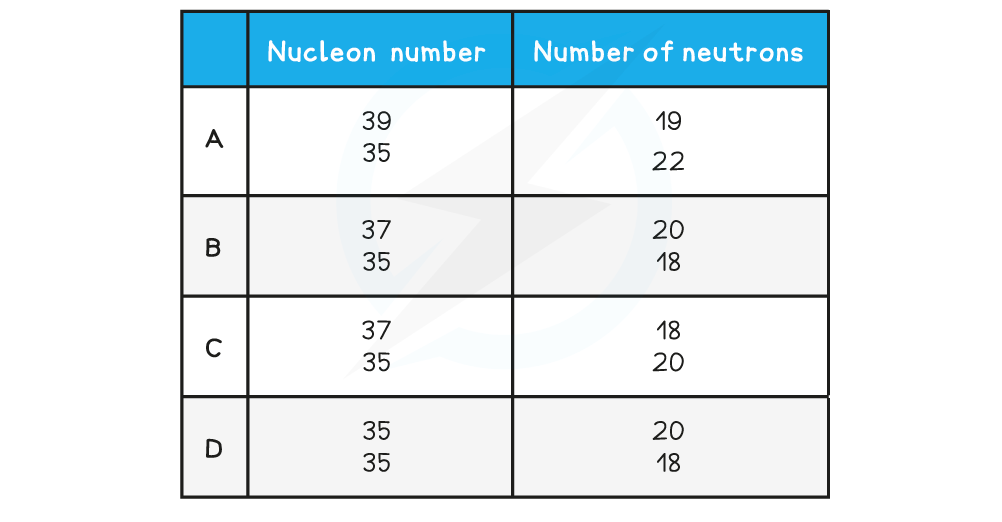
Which row is correct?
ANSWER: B
Step 1: Properties of isotopes
Isotopes are nuclei with the same number of protons but different number of neutrons
The nucleon number is the sum of the protons and neutron
Therefore, an isotope has a different nucleon number too
Step 2: Calculate protons in the first nucleus
Nucleon number: 37
Neutrons: 20
Protons = 37 − 20 = 17
Step 3: Calculate protons in the second nucleus
Nucleon number: 35
Neutrons: 18
Protons = 35 − 18 = 17
Step 4: Conclusion
Therefore, they have the same number of protons but different numbers of neutrons and are isotopes of each other
The correct answer is therefore option B
AZX Notation
- Atomic symbols are written in a specific notation called AZX notation

Atomic symbols, like the one above, describe the constituents of nuclei
- The top number A represents the nucleon number or the mass number
- Nucleon number (A) = total number of protons and neutrons in the nucleus
- The lower number Z represents the proton or atomic number
- Proton number (Z) = total number of protons in the nucleus
Note: In Chemistry the nucleon number is referred to as the mass number and the proton number as the atomic number. The periodic table is ordered by atomic number
Conservation of Nucleon Number & Charge
- Nuclear processes such as fission and fusion are represented using nuclear equations (similar to chemical reactions in chemistry)
- The number of protons and neutrons in atom is known as its constituents
- For example:

Nuclear fission equation
- The above equation represents a fission reaction in which a Uranium nucleus is hit with a neutron and splits into two smaller nuclei – a Strontium nucleus and Xenon nucleus, releasing two neutrons in the process
- In nuclear equations, the nucleon number and charge are always conserved
- This means that the sum of the nucleons and charge on the left hand side must equal the sum of the number of nucleons and charge on the right hand side
- In the above equation, the sum of the nucleon (top) numbers on both sides are equal
235 + 1 = 236 = 90 + 144 + 2 × 1
- The same is true for the proton (bottom) numbers
92 + 0 = 92 = 38 + 54 + 2 × 0
- By balancing equations in this way, you can determine the nucleon, proton number or the number of missing elements
- For example:
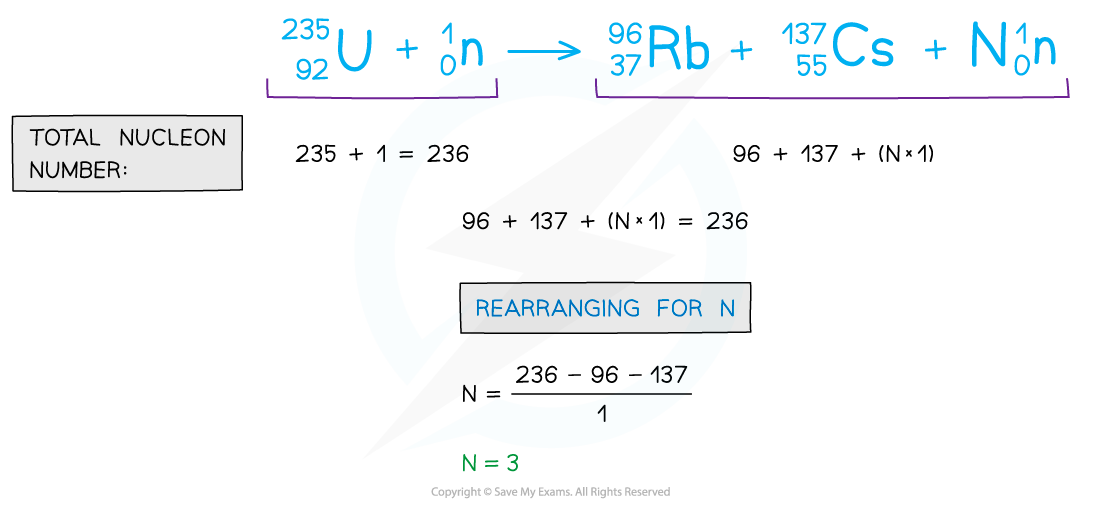
Balancing the number of nucleons shows that 3 neutrons must be released in the reaction
Worked Example
When a californium atom reacts with an unknown element X, the following reaction occurs.
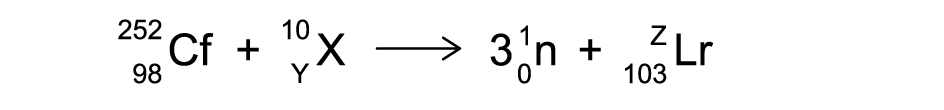
Determine the values of Y and Z.
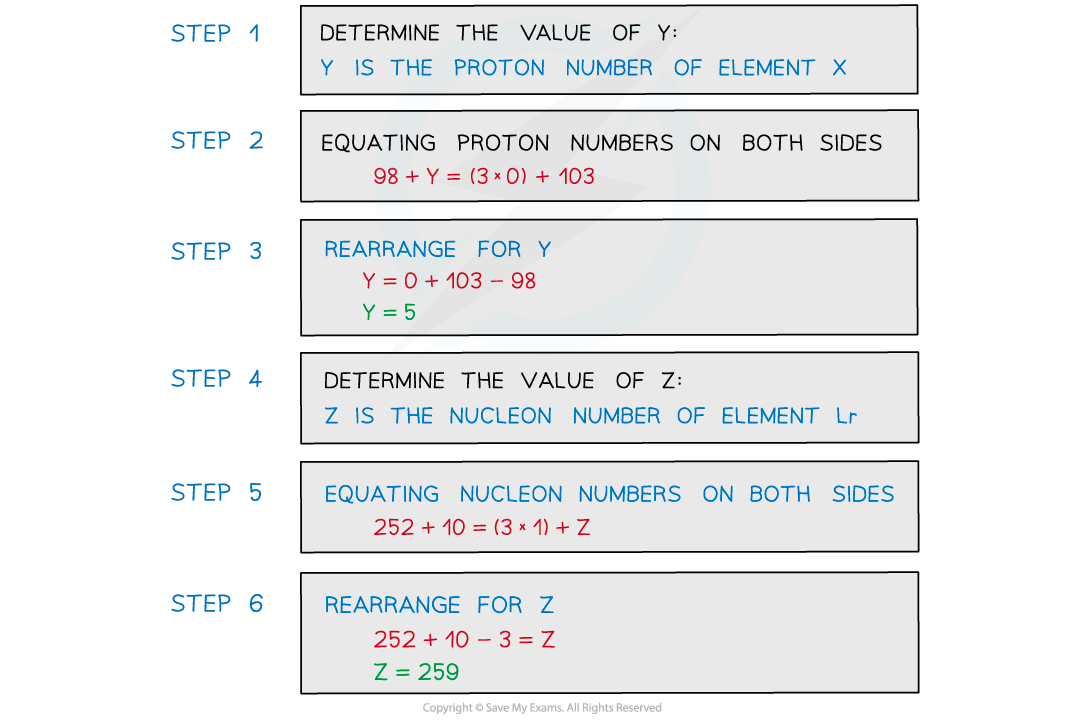
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









