The Esters
Making Esters
- Alcohols and carboxylic acids react to make esters in esterification reactions
- Esters are compounds with the functional group R-COO-R
- Esters are sweet-smelling oily liquids used in food flavourings and perfumes
- Ethanoic acid will react with ethanol in the presence of concentrated sulfuric acid (catalyst) to form ethyl ethanoate:
CH3COOH + C2H5OH → CH3COOC2H5 + H2O
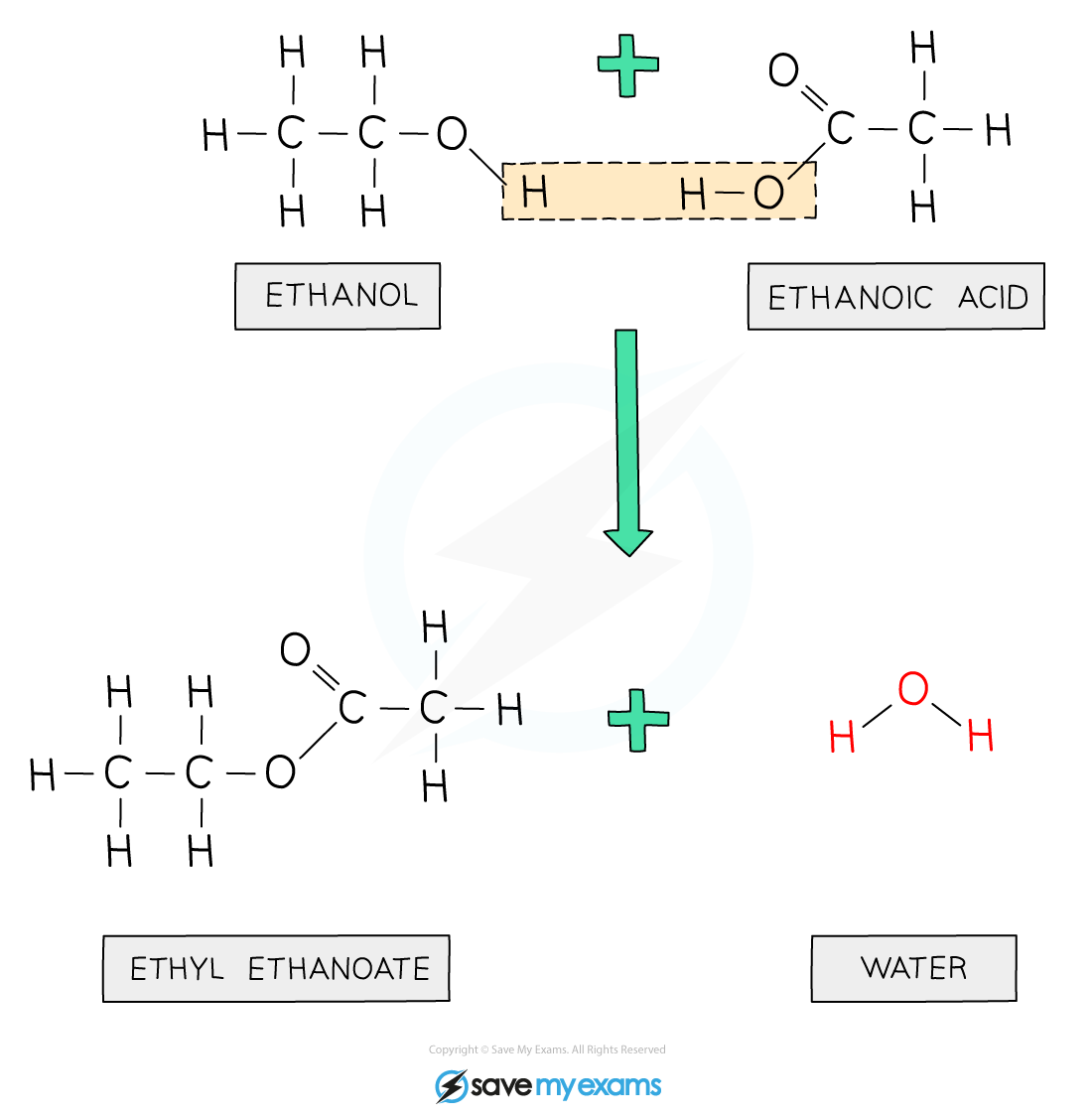 Diagram showing the formation of ethyl ethanoate
Diagram showing the formation of ethyl ethanoate
Naming Esters
- An ester is made from an alcohol and carboxylic acid
- The first part of the name indicates the length of the carbon chain in the alcohol, and it ends with the letters ‘- yl’
- The second part of the name indicates the length of the carbon chain in the carboxylic acid, and it ends with the letters ‘- oate’
- e.g. the ester formed from pentanol and butanoic acid is called pentyl butanoate
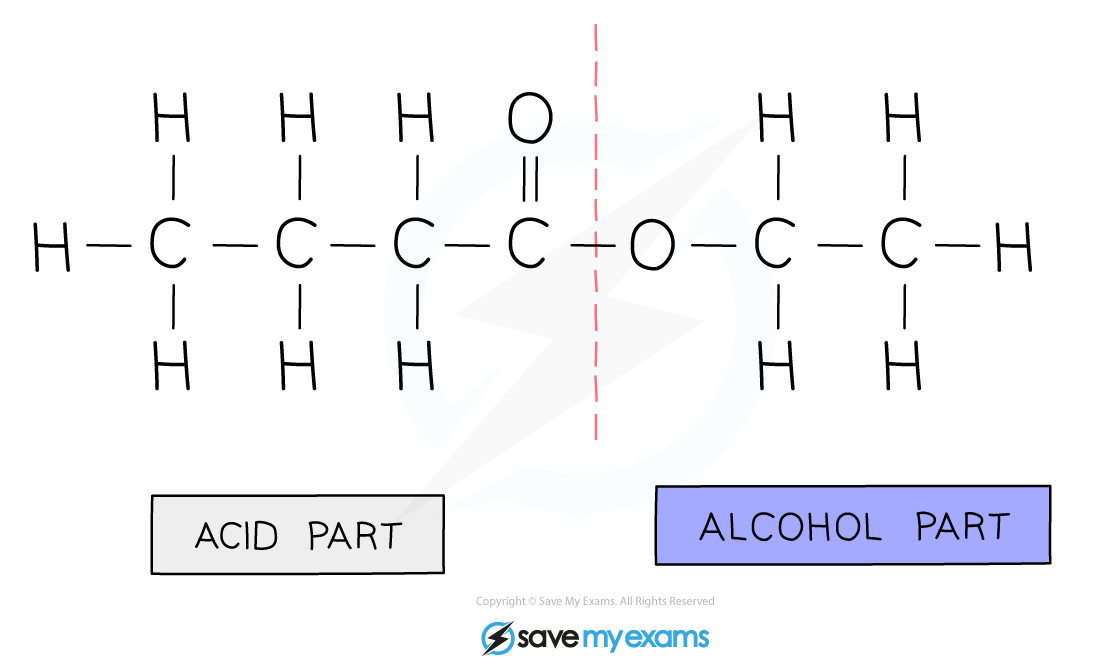 Diagram showing the origin of each carbon chain in ester
Diagram showing the origin of each carbon chain in ester
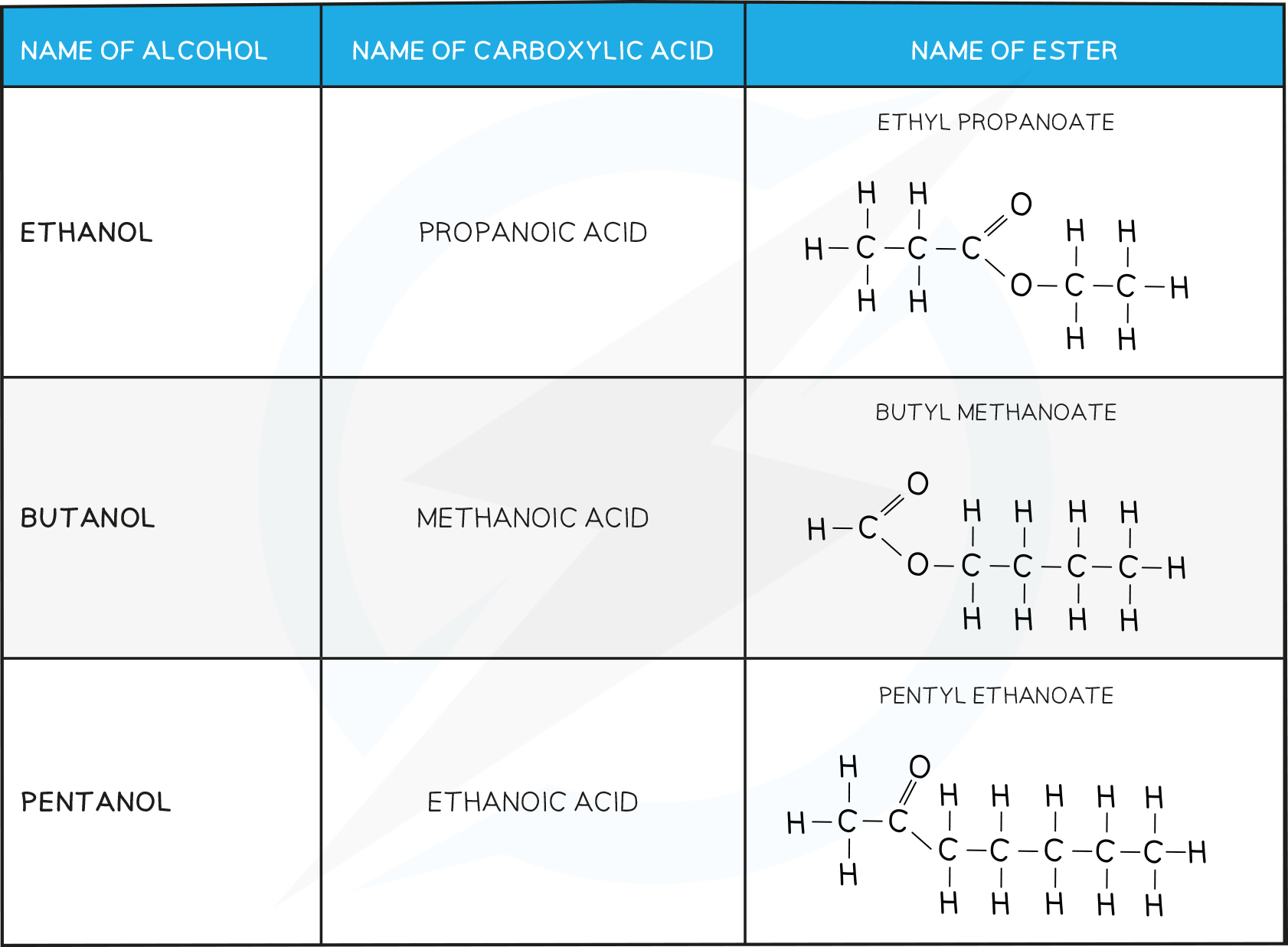
- Some examples of common esters:
Examples of Esters Table