- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel IGCSE Chemistry 复习笔记 3.3.3 The Position of Equilibrium
Edexcel IGCSE Chemistry 复习笔记 3.3.3 The Position of Equilibrium
Factors that Affect the Position of Equilibrium
- When a change is made to the conditions of a system at equilibrium, the system automatically moves to oppose the change
- This is known Le Chatelier’s Principle, but you are not required to know the name for the exam
- The principle is used to predict changes to the position of equilibrium when there are changes in temperature or pressure
Effects of Temperature

ExampleIodine Monochloride reacts reversibly with Chlorine to form Iodine Trichloride
ICl + Cl2 ⇌ ICl3
Dark Brown Yellow
When the equilibrium mixture is heated, it becomes dark brown in colour. How do we know whether the backward reaction is exothermic or endothermic?
- Equilibrium has shifted to the left as the colour dark brown means that more of ICI is produced
- Increasing temperature moves the equilibrium in the endothermic direction
- So the backward reaction is endothermic
Effects of Pressure

ExampleNitrogen Dioxide can form Dinitrogen Tetroxide, a colourless gas
2NO2 ⇌ N2O4
Brown Gas Colourless Gas
What is the effect of an increase in pressure on the position of equilibrium?
-
- Number of molecules of gas on the left = 2
- Number of molecules of gas on the right = 1
- An increase in pressure will cause equilibrium to shift in the direction that produces the smaller number of molecules of gas
- So equilibrium shifts to the right
Exam Tip
When the conditions at equilibrium are changed, the system always responds by doing the opposite.
Catalysts & Dynamic Equilibrium
Effect of catalyst on equilibrium position
- The presence of a catalyst does not affect the position of equilibrium but it does increase the rate at which equilibrium is reached
- This is because the catalyst increases the rate of both the forward and backward reactions by the same amount (by providing an alternative pathway requiring lower activation energy)
- As a result, the concentration of reactants and products is nevertheless the same at equilibrium as it would be without the catalyst
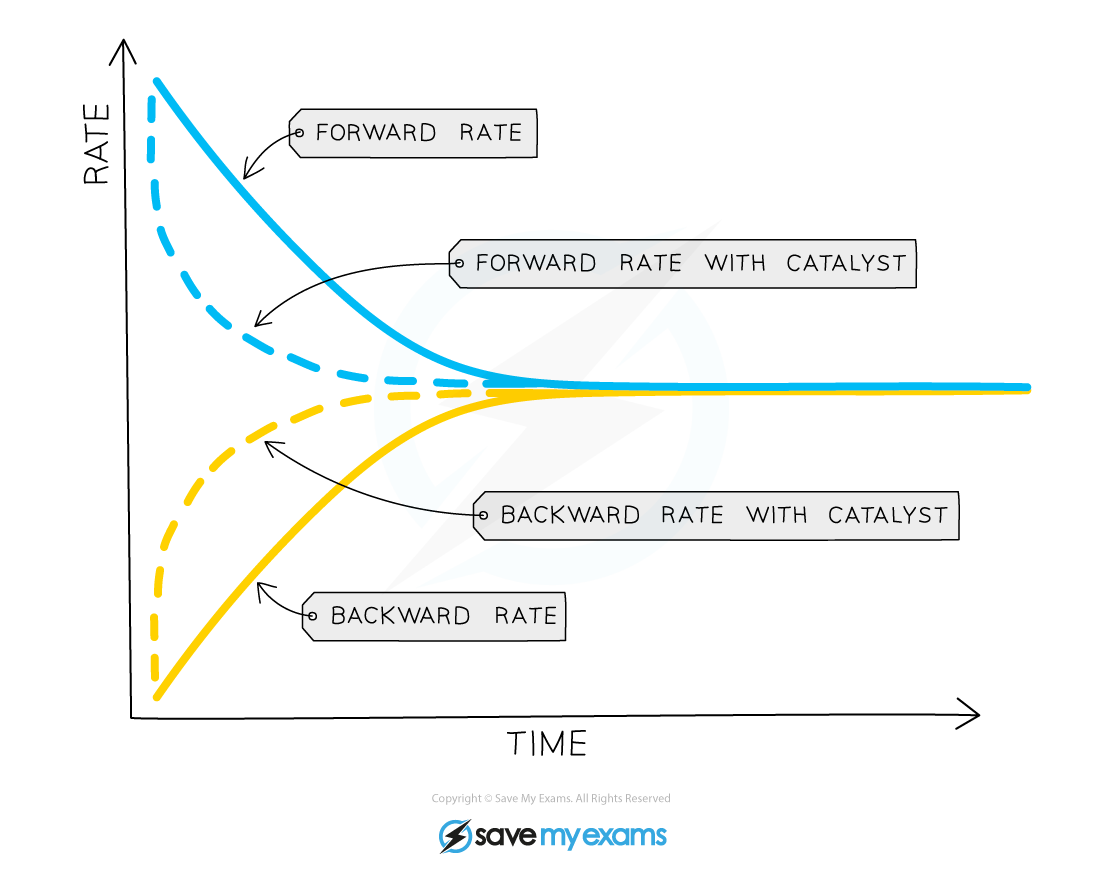 Diagram showing the effect of catalyst on equilibrium position
Diagram showing the effect of catalyst on equilibrium position
转载自savemyexams


最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








