- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记7.6.5 Relative Basicity of Ammonia, Ethylamine & Phenylamine
Relative Basicity of Aqueous Ammonia, Ethylamine & Phenylamine
- Ammonia and amines act as bases as they can donate their lone pair of electrons to form a dative covalent bond with a proton
- The basicity of the amines depends on how readily available their lone pair of electrons is
- Electron-donating groups (such as alkyl groups) increase the electron density on the nitrogen atom and cause the lone pair of electrons to become more available for dative covalent bonding
- The amine becomes more basic
- Delocalisation of the lone pair of electrons into an aromatic ring (such as a benzene ring) causes the lone pair of electrons to become less available for dative covalent bonding
- The amine becomes less basic
Comparing basicity of ammonia, ethylamine & phenylamine
- The order of basicity of ammonia, ethylamine and phenylamine is as follows:
Ethylamine > ammonia > phenylamine
STRONGEST BASE WEAKEST BASE
- This trend can be explained by looking at the groups attached to the amine (-NH2) group
- In ethylamine, the electron-donating alkyl group donates electron density to the nitrogen atom causing its lone pair to become more available to form a dative covalent bond with a proton
- Ammonia lacks an electron-donating group hence it is less basic than ethylamine however it is more basic than phenylamine as the lone pair on the nitrogen is not delocalised
- In phenylamine the lone pair of electrons overlap with the conjugated system on the benzene ring and become delocalised; As a result, the lone pair of electrons become less readily available to form a bond with a proton
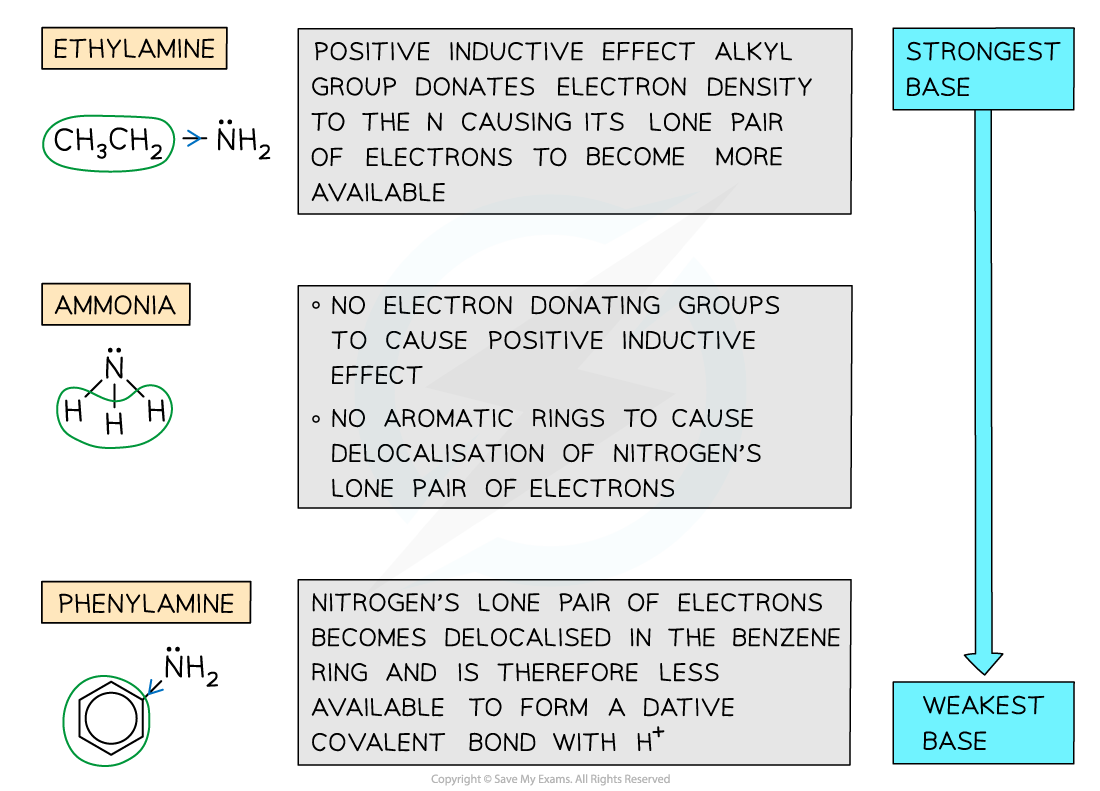
Trends in the basicity of ammonia, ethylamine, and phenylamine
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









