- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记7.6.3 Basicity of Amines
Basicity of Aqueous Solutions of Amines
- The nitrogen atom in ammonia and amine molecules can accept a proton (H+ ion)
- They can therefore act as bases in aqueous solutions by donating its lone pair of electrons to a proton and form a dative bond
- For example, ammonia undergoes an acid-base reaction with dilute hydrochloric acid (HCl) to form a salt
NH3 + HCl → NH4+Cl-
base acid salt
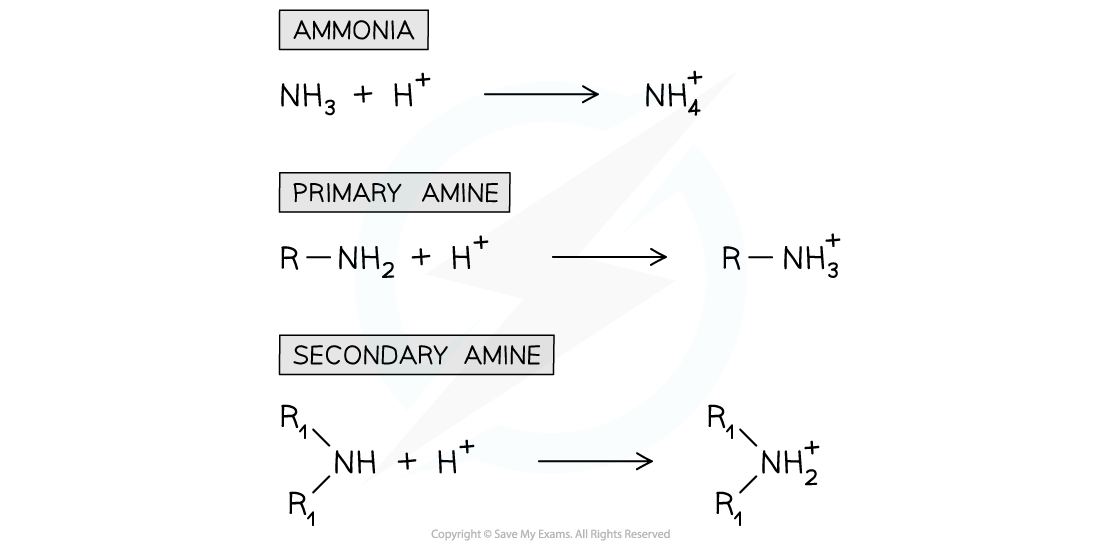
The nitrogen atom in ammonia and amines can donate its lone pair of electrons to form a bond with a proton and therefore act as a base
Strength of ammonia and amines as bases
- The strength of amines depends on the availability of the lone pair of electrons on the nitrogen atom to form a dative covalent bond with a proton
- The more readily this lone pair of electrons is available, the stronger the base is
- Factors that may affect the basicity of amines include:
- Positive inductive effect - Some groups such as alkyl groups donate electron density to the nitrogen atom causing the lone pair of electrons to become more available and therefore increasing the amine’s basicity
- Delocalisation - The presence of aromatic rings such as the benzene ring causes the lone pair of electrons on the nitrogen atom to be delocalised into the benzene ring
- The lone pair becomes less available to form a dative covalent bond with ammonia and hence decreases the amine’s basicity
- For example, ethylamine (which has an electron-donating ethyl group) is more basic than phenylamine (which has an electron-withdrawing benzene ring)
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









