- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记7.5.2 Reactions of Carboxylic Acids
Reactions of Carboxylic Acids to Produce Acyl Chlorides
- Acyl chlorides are compounds with the functional group -COCl
- They look similar in structure to carboxylic acids but have a Cl atom instead of an -OH group attached to the carbonyl (C=O)
- Acyl chlorides are more reactive than their corresponding carboxylic acids and are therefore often used as starting materials in the production of organic compounds such as esters
- They can be prepared from the reaction of carboxylic acids with:
- Solid phosphorus(V) chloride (PCl5)
- Liquid phosphorus(III) chloride (PCl3) and heat
- Liquid sulfur dichloride oxide (SOCl2)
- For example, the acyl chloride ethanoyl chloride can be formed from ethanoic acid in the above reactions
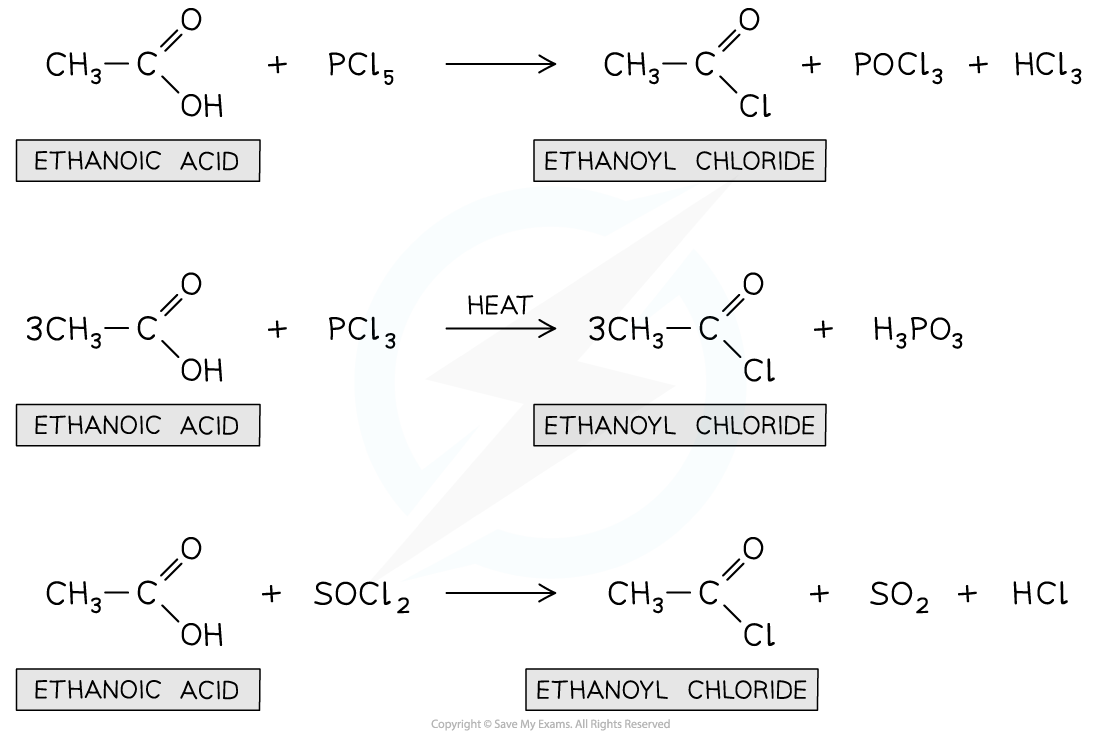
Production of acyl chlorides from their corresponding carboxylic acids
Further Oxidation of Carboxylic Acids
- Carboxylic acids can be formed from the oxidation of primary alcohols
- The primary alcohols are firstly oxidised to aldehydes and then further oxidised to carboxylic acids
- Some carboxylic acids can get even further oxidised
Methanoic acid
- Methanoic acid is a strong reducing agent and gets further oxidised to carbon dioxide (CO2)
- The oxidation of methanoic acid can occur by:
- Warming methanoic acid with mild oxidising agents such as Fehling’s or Tollens’ reagent
- In a Fehling’s solution, the Cu2+ ion is reduced to Cu+ ion which precipitates as red Cu2O
- With Tollens’ reagent, the Ag+ is reduced to Ag
- Using stronger oxidising agents such as acidified KMnO4or acidified K2Cr2O7
- The purple KMnO4 solution turns colourless as Mn7+ ions are reduced to Mn2+ ions
- The orange K2Cr2O7 solution turns green as the Cr6+ ions are reduced to Cr3+ ions
- Warming methanoic acid with mild oxidising agents such as Fehling’s or Tollens’ reagent
Ethanedioic acid
- Another carboxylic acid that can get further oxidised is ethanedioic acid
- A strong oxidising agent such as warm acidified KMnO4is required for the oxidation of ethanedioic acid to carbon dioxide
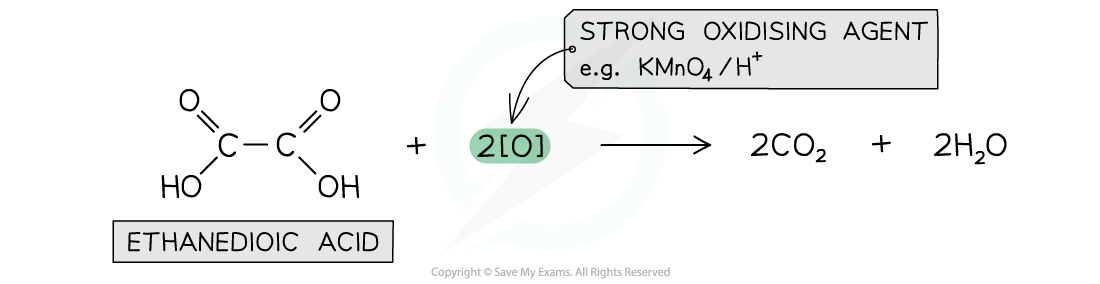
Ethanedioic acid is a dicarboxylic acid that can get further oxidised to carbon dioxide
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









