- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记7.4.5 Nitration & Bromination of Phenol
Nitration & Bromination of Phenol
- Compared to benzene, phenol reacts more readily with electrophiles
- This is because one of the lone pairs of electrons on the oxygen atom in phenol overlaps with the π bonding system of the benzene ring
- As a result, there is now an increased electron density in the ring
- The electron-donating -OH group in phenol, therefore, activates the benzene ring and directs incoming electrophiles to the 2, 4, and 6 positions
- The increased reactivity of phenol means that different reagents and conditions are used for electrophilic substitution reactions of phenols compared to benzene
Nitration
- Nitration is an example of an electrophilic substitution reaction
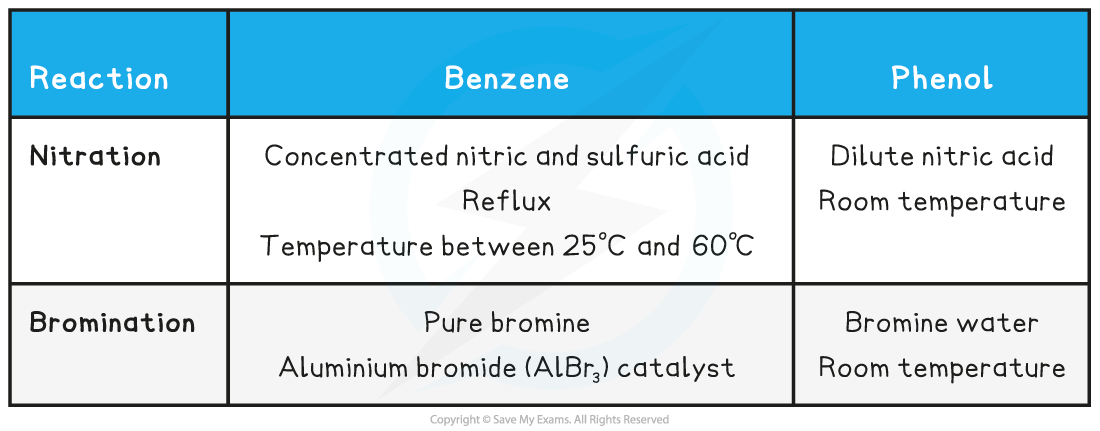
- The nitration of benzene requires a mixture of concentrated nitric acid (HNO2) and sulfuric acid (H2SO4) refluxed with benzene between 25 oC and 60 oC
- Since phenol is more reactive, nitration can occur under milder conditions by reacting it with dilute nitric acid at room temperature
- If concentrated nitric acid is used, 2,4,6-trinitrophenol is formed
Bromination
- Bromination is another example of an electrophilic substitution reaction
- Benzene will undergo bromination only when reacted with pure bromine (not a solution) and in the presence of an anhydrous aluminium bromide (AlBr3) catalyst at room temperature
- Phenol on the other hand readily reacts with bromine water in the absence of a catalyst
Reagents & conditions for nitration and bromination of phenol & benzene table
Directing Effects of Hydroxyl Group on Phenol
- Phenols consist of a hydroxyl (-OH) group attached to a benzene ring
- The oxygen atom in this hydroxyl group donates electron density into the ring
- One of the lone pairs of the oxygen atom overlaps with the π system of the benzene ring and become delocalised causing an increased electron density in the aromatic ring
- Due to the increased electron density, the benzene ring is now more likely to undergo electrophilic attack and becomes activated
- The incoming electrophiles are directed by the hydroxyl group of the phenol to the 2, 4, and 6 positions
- An example is the bromination of phenol
- The bromine acts as an electrophile and substitutes a hydrogen atom in the benzene ring
- The substitution of the hydrogen atom can occur on the 2, 4, or 6 positions
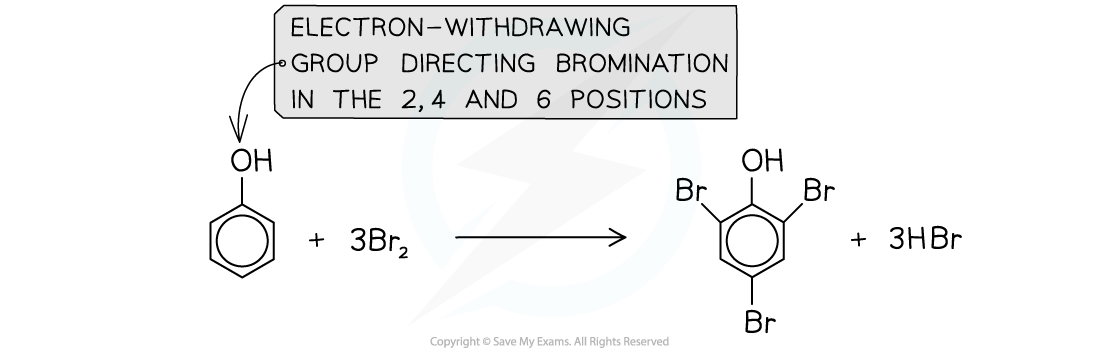
The hydroxyl group in phenol directs bromination in the 2, 4, and 6 positions
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









