- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记7.4.4 Acidity of Phenols
Acidity of Phenols
- Although phenol compounds contain an alcohol (-OH) group, they are weakly acidic
- This is due to the delocalisation of one of the lone pairs from the oxygen atom into the aromatic ring
- This increases the electron density of the ring and increases the acidic behaviour
Delocalisation of charge density
- The conjugate base of phenol is the phenoxide ion
- In the phenoxide ion, the negative charge on the oxygen is spread out over the entire ion
- This is possible as one of the lone pairs on the oxygen atom overlaps with the delocalised π system of the ring
- Because of this delocalisation, there is less charge density on the oxygen atom
- The H+ ions are therefore not strongly attracted to the phenoxide ion and are less likely to reform the phenol molecule
- This means that phenol is more likely to lose a proton (and act as an acid) rather than to gain a proton (and act as a base )
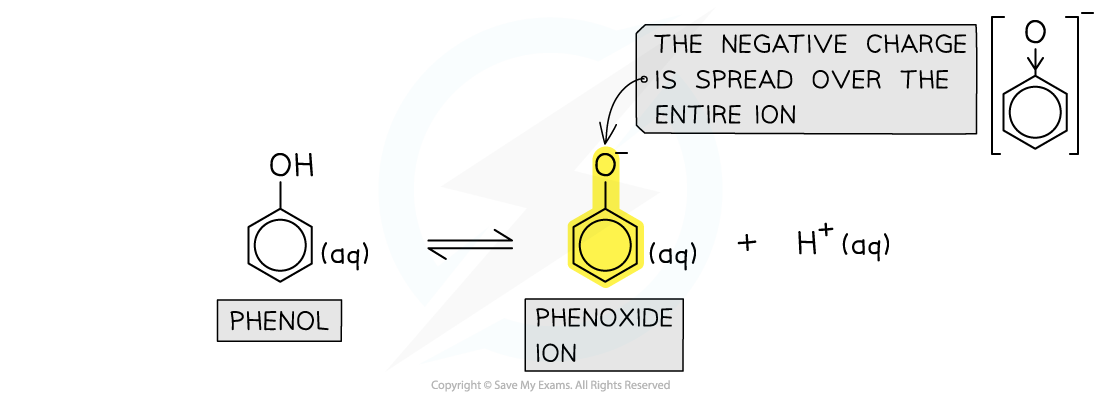
The negative charge is spread over the ion, causing the electrons to become less available for bonding with an incoming proton
Stability of the conjugate base
- Phenol ionises to form a more stable negative phenoxide ion with its negative charge spread out
- This means that phenol is more likely to undergo ionisation
- The equilibrium position, therefore, lies further to the right and a higher proportion of phenol molecules donate a proton compared to for example water and ethanol
- The phenol compound is, therefore, more likely to act as an acid rather than a base

Since the phenoxide ion formed from the ionisation of phenol is more stable than phenol itself, the equilibrium position lies further to the right-hand-side and phenol is more likely to act as an acid rather than a base
Relative Acidities of Water, Phenol & Ethanol
- The pKa is a measure of the acidity of a substance
- The values of water, phenol, and ethanol show that phenol is a stronger acid than ethanol and water
Relative acidity of ethanol, water & phenol table

- The order of acidity can be explained by looking at their conjugate bases which are formed from the dissociation of the compounds
Delocalisation of charge density
- In the phenoxide ion (which is the conjugate base of phenol) the charge density on the oxygen atom is spread out over the entire ion
- As a result, the electrons on the oxygen atom are less available for bond formation with a proton (H+ ion)
- The conjugate base of ethanol is the ethoxide ion
- The ethyl group in the ion is an electron-donating group that donates electron density to the oxygen atom
- As a result, the electron density on the oxygen atom is more readily available for bond formation with an H+ ion
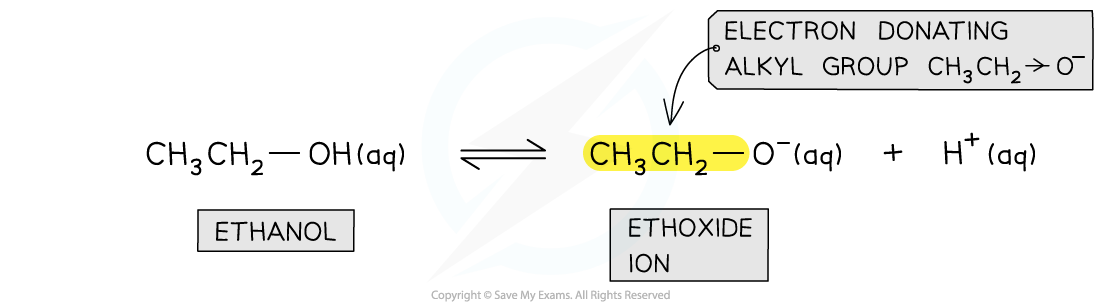
The electron-donating alkyl group in the ethoxide ion concentrates charge density on the oxygen atom which can more easily bond an H+ ion
- The conjugate base of water is the hydroxide ion
- Since the charge density of the oxygen atom cannot become delocalised over a ring, the hydroxide ion more readily accepts an H+ ion compared to the phenoxide ion
- Water is, therefore, a stronger base compared to phenol
- However, as there are no electron-donating alkyl groups, less negative charge is concentrated on the oxygen atom which therefore less readily accepts an H+ ion compared to the ethoxide ion
- Water is, therefore, a weaker base compared to ethanol

The hydroxide ion lacks an aromatic ring and electron-donating alkyl groups so water is a stronger base than phenol but a weaker base than ethanol
- Therefore, the position of equilibrium lies:
- Further to the right-hand side favouring the dissociated phenoxide ions
- Further to the left-hand side favouring the undissociated ethoxide and hydroxide ions
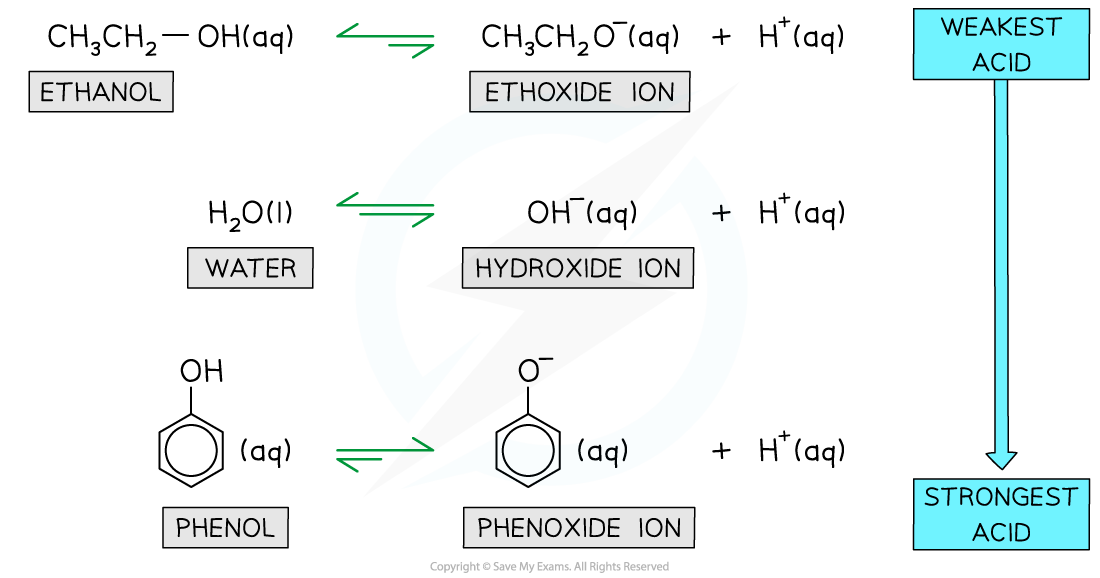 Relative equilibrium positions for the dissociation of ethanol, water, and phenol
Relative equilibrium positions for the dissociation of ethanol, water, and phenol
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









