- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记7.3.2 Reactivity of Halogenoarenes
Difference in Reactivity of Halogenoalkanes & Halogenoarenes
- Halogenoarenes are very unreactive compared to halogenoalkanes
- The difference in reactivity between the two compounds is because of the carbon-halogen bond strengths
Halogenoalkanes
- The halogenoalkane chloroethane can take part in nucleophilic substitution reactions
- A nucleophile, such as a hydroxide (OH-) ion, will attack the slightly positive carbon atom
- A covalent bond is formed between that carbon atom and the nucleophile which causes the carbon-halogen bond to break
- Overall, the halogen is replaced by the nucleophile
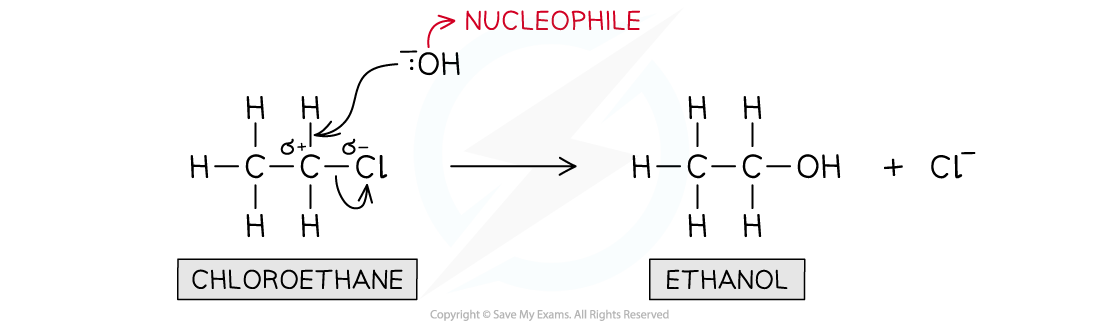
Halogenoalkanes readily undergo nucleophilic substitution reactions
Halogenoarenes
- Halogenoarenes, such as chlorobenzene, do not readily undergo nucleophilic substitution reactions
- Only under extremely harsh conditions, such as temperatures of 200 oC and a pressure of 200 atmospheres, will the chlorine in chlorobenzene get replaced by a nucleophile such as a hydroxide (OH-) ion
- This is because the carbon-chlorine bond is very strong and cannot be easily broken
- One of the lone pairs of electrons on the chlorine will interact with the π system of the ring
- This causes the carbon-chlorine bond to have a partial double-bond character, which strengthens the bond
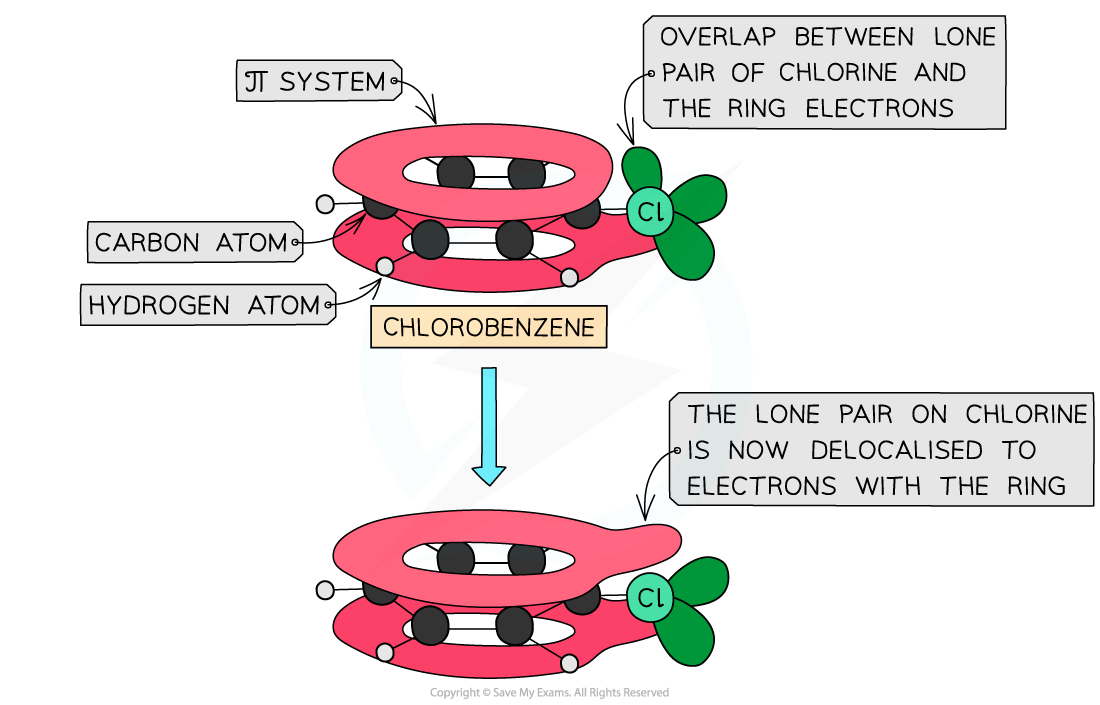
The carbon-chlorine bond is very strong, as it has partial double-bond character
- The unreactivity of halogenoarenes can therefore be explained by the delocalisation of a lone pair on the halogen over the benzene
- This causes additional stabilisation of the system and strengthens the carbon-halogen bond, which affects the reactions that halogenoarenes will undergo
- It gets harder to break the carbon-halogen bond in halogenoarenes, which decreases reactivity
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









