- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记7.1.5 Shape of Aromatic Molecules
Shape of Benzene & Aromatic Molecules
- Aromatic molecules consist of one or more rings with conjugated π systems
- Conjugated π systems arise from alternating double and single bonds in which the electrons are delocalised
- Aromatic compounds are called ‘aromatic’ as they often have pleasant odours
Examples of aromatic compounds including benzene table
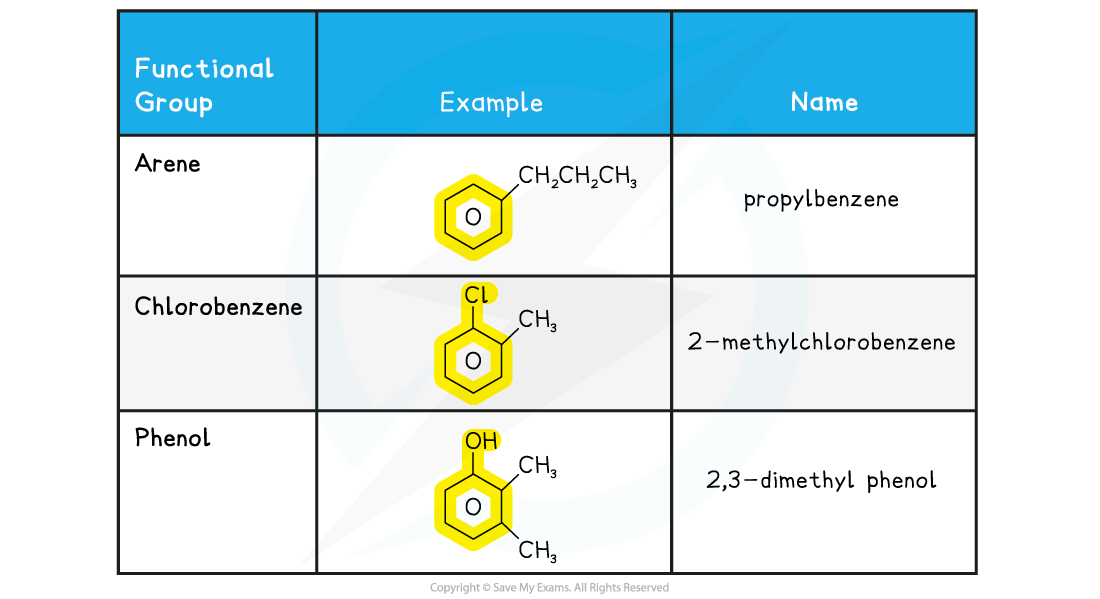
Shape of benzene & aromatic compounds
- Benzene and other aromatic compounds contain sp2 hybridised carbons as two of their p orbitals have mixed with an s orbital
- This means that each carbon atom in benzene and other aromatic compounds has one p orbital
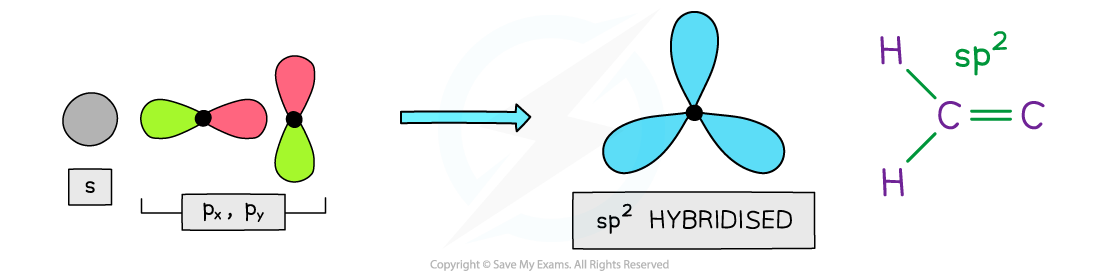
The carbon atoms in aromatic compounds are sp2 hybridized as two of their p orbitals mix with an s orbital
- Each carbon atom in the ring forms three σ bonds using the sp2 orbitals
- The remaining p orbital overlaps laterally with p orbitals of neighbouring carbon atoms to form a π bond
- This extensive sideways overlap of p orbitals results in the electrons being delocalised and able to freely spread over the entire ring
- Benzene and other aromatic compounds are regular and planar compounds with bond angles of 120o
- The delocalisation of electrons means that all of the carbon-carbon bonds in these compounds are identical and have both single and double bond character
- The bonds all being the same length is evidence for the delocalised ring structure of benzene
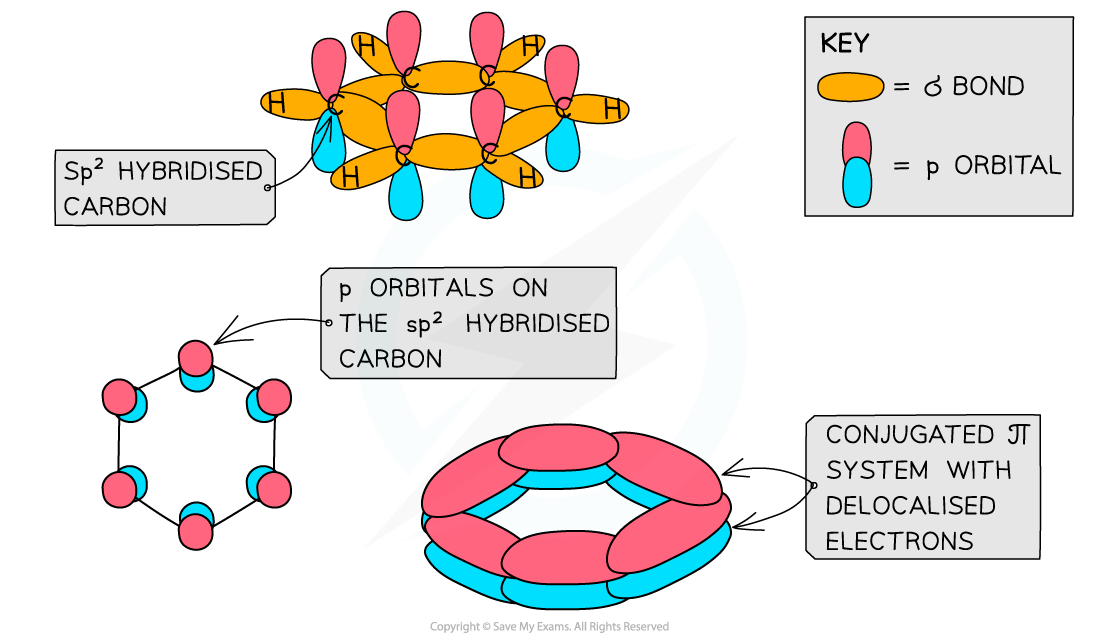
Like other aromatic compounds, benzene has a planar structure due to the sp2 hybridisation of carbon atoms and the conjugated π system in the ring
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









