- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记6.3.3 Redox Systems
Redox Systems: Ferrous & Permanganate
- The oxidation states of transition element ions can change during redox reactions
- A species will either be oxidised or reduced, depending on what reaction is occurring
- To find the concentration of specific ions in solution, a titration can be performed
- There are three particular redox titrations that need to be learnt:
- Iron (II) (Fe2+) and permanganate (MnO4-) in acid solution given suitable data
- Permanganate (MnO4-) and ethanedioate (C2O42-) in acid solution given suitable data
- Copper (II) (Cu2+) and iodide (I-) given suitable data
Reaction of MnO4- & Fe2+ in acid
- The concentration of Fe2+ ions can be determined by titrating a known volume of Fe(II) ions with a known concentration of MnO4- ions
- During the reaction of MnO4- with Fe2+, the purple colour of the manganate(VII) ions disappears
- The end-point is when all of the Fe2+ ions have reacted with the MnO4- ions, and the first trace of a permanent pink colour appears in the flask
- At this point, the MnO4- is very slightly in excess
- The two half-reactions that are involved in this redox reaction are as following:
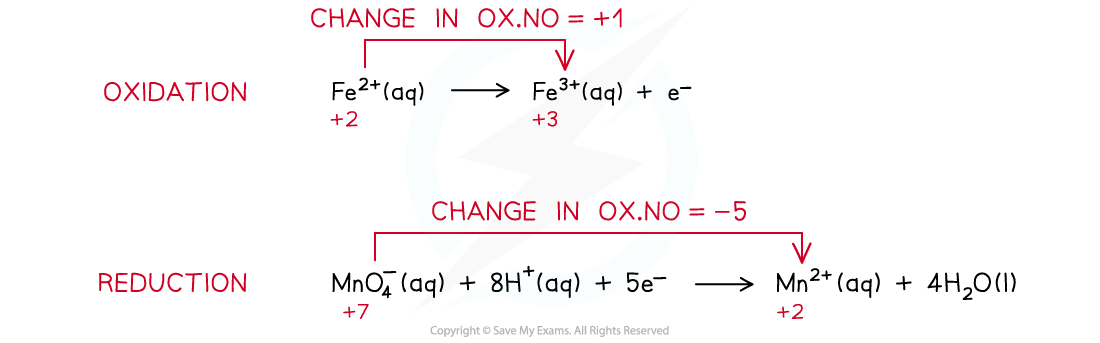
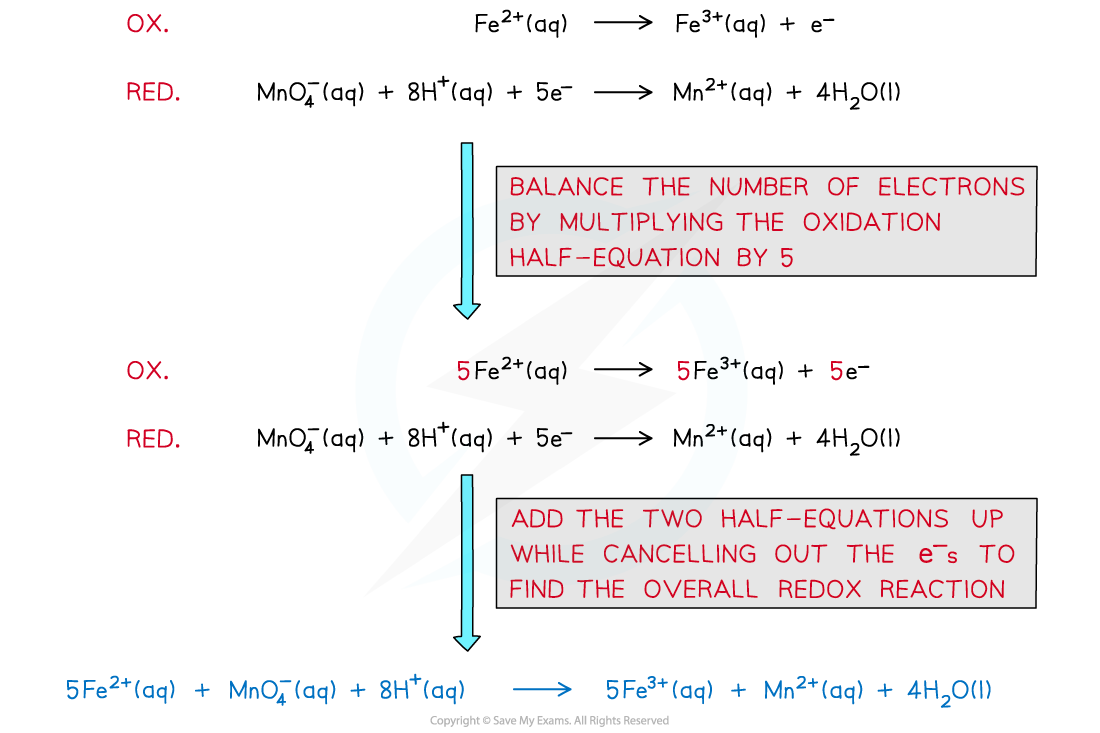
- The half equations are combined to get the overall equation
Redox Systems: Permanganate & Oxalate
- The second redox titration involving transition element ions that needs to be learned, is the titration of permanganate (MnO4-) and ethanedioate, sometimes known as oxalate (C2O42-) in acid solution given suitable data
Reaction of MnO4- & C2O42- in acid
- The reaction of MnO4- with ethanedioate, C2O42- is an example of a redox reaction in which the ethanedioate ions (C2O42-) get oxidised by manganate(VII) (MnO4-) ions
- A titration reaction can be carried out to find the concentration of the toxic ethanedioate ions
- As before, the end point is when all of the ethanedioate ions have reacted with the MnO4- ions, and the first permanent pink colour appears in the flask
- At this point, the MnO4- is very slightly in excess
- The two half-reactions that are involved in this redox reaction are as following:
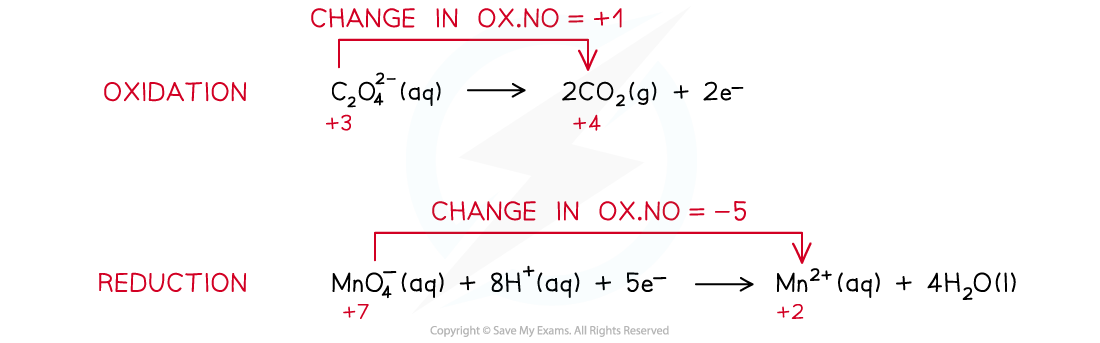
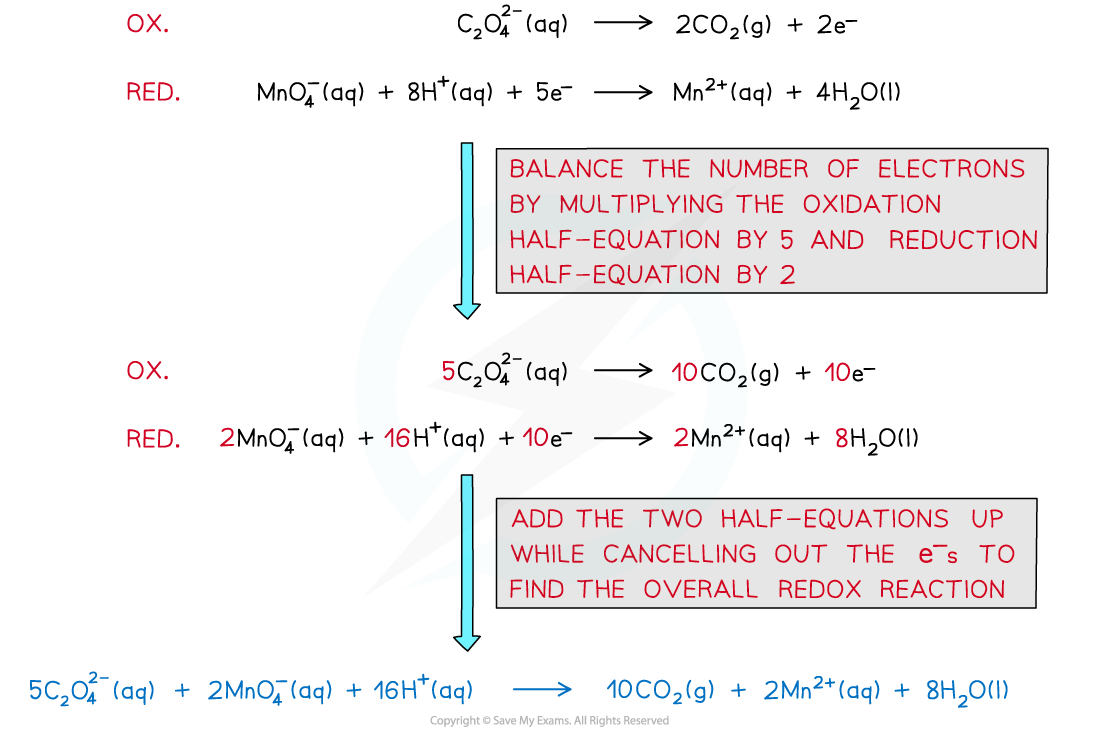
- The half equations are combined to get the overall equation
- This is an example of an autocatalysis reaction
- This means that the reaction is catalysed by one of the products as it forms
- In this reaction, the Mn2+ ions formed act as the autocatalyst
- The more Mn2+ formed, the faster the reaction gets, which then forms even more Mn2+ ions and speeds the reaction up even further
- Transition element ions can act as autocalysts as they can change their oxidation states during a reaction
Redox Systems: Cupric & Iodide
- The third redox titration involving transition metal ions that needs to be learnt is the titration between copper (II) ions (Cu2+) - sometimes known as cupric ions - and iodide ions (I-)
Reaction of Cu2+ & I-
- The reaction of Cu2+ with I- is an example of a redox reaction in which the copper ions (Cu2+) oxidise the iodide ions (I-) and as a result are themselves reduced
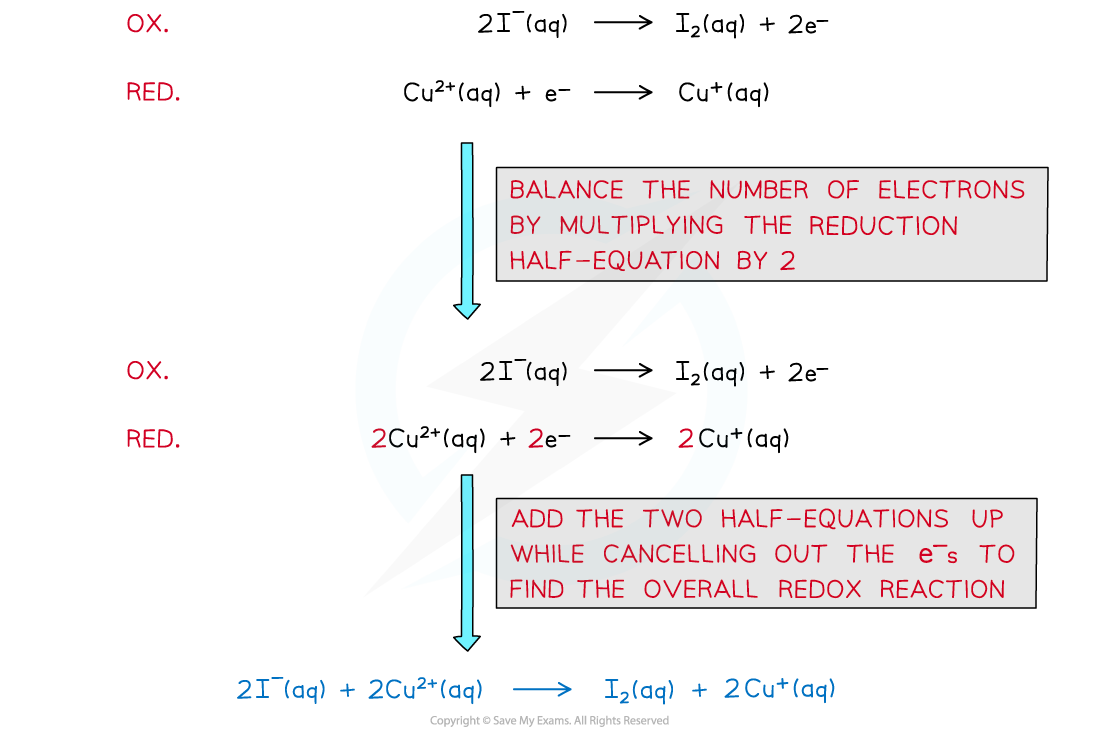
- The two half-reactions that are involved in this redox reaction are as follows:
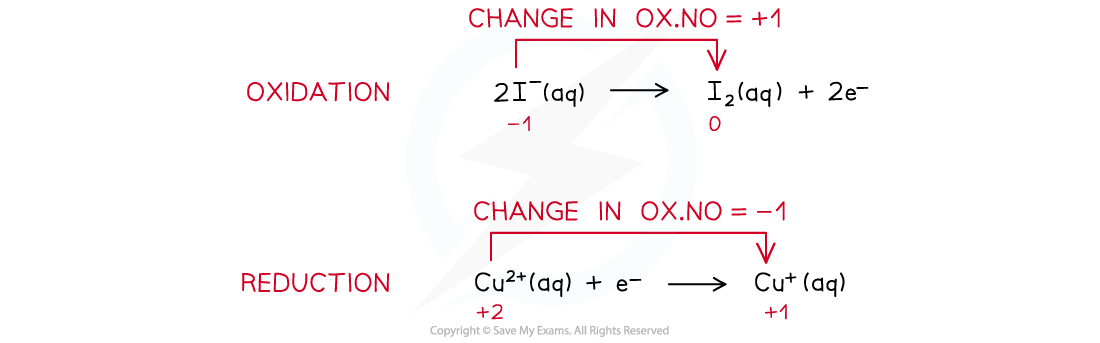
- The half equations could be combined to get this overall equation
- When excess iodide ions are reacted with Cu(II), a precipitate of copper(I) iodide and iodine is formed
2Cu2+ (aq) + 4I-(aq) → I2(aq) + 2CuI (s) Reaction 1
- A titration reaction can be carried out to find an unknown concentration of the copper(II) solution
- This is done by finding the amount of iodine which is liberated during the reaction, through a titration
- Step 1: A known concentration of sodium thiosulfate solution is added to the mixture formed in Reaction 1 from a burette
- Step 2: The I2 formed in Reaction 1 will react with the thiosulfate ions
I2 (aq) + 2S2O32- (aq) → 2I- (aq) + S4O62- (aq) Reaction 2
- Step 3: As the iodine is used, up the brownish colour of the solution gets lighter
- Step 4: When most of the iodine colour is gone, starch is added, which turns deep blue/black with the remaining I2 (aq)
- Step 5: Titrate further until the blue/black colour disappears, i.e. when all of the iodine has reacted
- By knowing the number of moles of thiosulfate ions added in the titration, you can use the molar ratios from the reaction equations and work backwards to calculate the number of moles of Cu(II)
- Step 6: Look at Reaction 2: it can be concluded that half the number of moles of I2 reacts when compared to the moles of thiosulfate that react
- Step 7: Now look at Reaction 1: the number of moles of I2 which reacts in Reaction 2, is the moles formed in Reaction 1. The number of moles of Cu(II) is twice that of I2 (aq) (i.e. the same number of moles as thiosulfate ions added in the titration)
- Step 8: Divide the number of moles of Cu(II) by the volume in dm3 to get the concentration of Cu(II)
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









