- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记6.3.2 Predicting Feasibility of Redox Reactions
Feasibility of Redox Reactions Using Standard Electrode Values
- Transition elements can form ions with various oxidation states
- The change in their oxidation states involves the transfer of electrons
- Transition elements are often involved in redox reactions
- A redox reaction is a reaction in which one species is oxidised (loses electrons) and another is reduced (gains electrons)
- The standard electrode potentials (Eꝋ) of the two species can be used to predict the feasibility of redox reactions involving transition elements and their ions
Predicting feasibility of redox reactions
- The standard electrode potential (Eꝋ) of a species gives an indication of how well it can be reduced
- In the exam, you will be provided with a half equation and the standard electrode potential (Eꝋ)
- The half equations are always written as a reduction equation
- They are equilibrium reactions, as they demonstrate the equilibrium reached when the species in the equation gains electrons at the same rate as it is losing electrons
- The more positive the standard electrode potential (Eꝋ) of a species is, the more readily that element will be reduced (gain electrons)
- This is always when compared to the standard hydrogen electrode
- The opposite is of course true; the more negative the standard electrode potential (Eꝋ) of a species is, the more readily that element will be oxidised (lose electrons)
- The feasibility of a reaction can be predicted using these values
- For example, the feasibility of Fe3+ being reduced to Fe2+ when reacted with Cu2+, can be predicted using their standard electrode potentials
Standard electrode potentials of Fe(III) & Cu(II) table
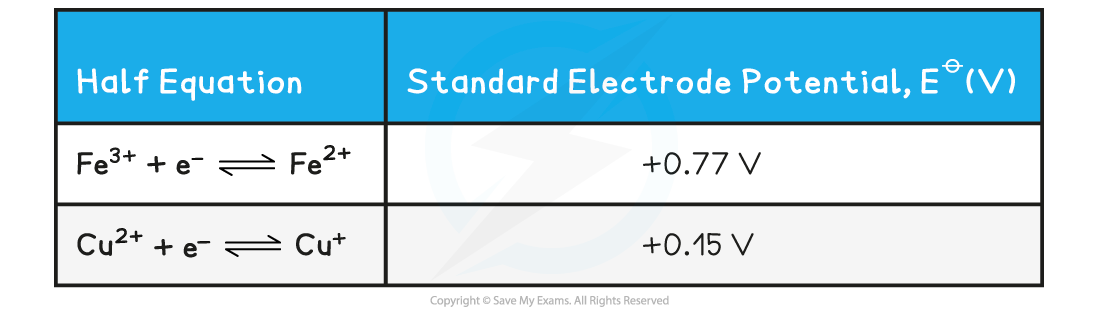
- The table above shows that yes, the reaction is feasible and Fe3+ is more likely to get reduced to Fe2+
- Fe3+ has a more positive standard electrode potential
- Fe3+ will gain electrons more readily than Cu2+
- Therefore, Fe3+ is the better oxidising agent
- The reaction for this half equation will therefore proceed in the forward direction (reduction)
- Since it is feasible that the Fe3+ will be reduced and this half equation will move in the forward direction, this means that the half equation for copper will move in the backward direction (oxidation)
- Cu2+ equation has a less positive (or more negative) standard electrode potential
- The Cu+ will therefore be oxidised to Cu2+
- The reaction for this half equation will therefore be in the reverse direction
- Combining these two half-equations to get the overall equation gives (after cancelling the electrons on both sides):
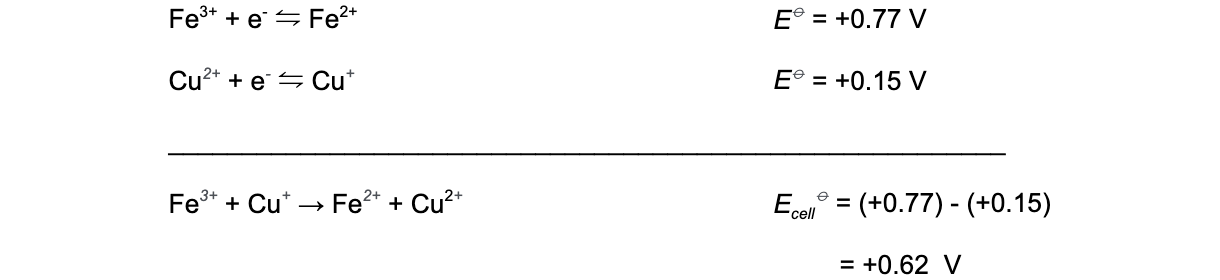
- The positive value of Ecellꝋ (+0.62 V) suggests that the reaction is likely to proceed
- The changes in the transition element ions’ oxidation states are therefore feasible
- Standard electrode potentials (Eꝋ) are only predictions about the feasibility of a reaction; they do not guarantee that a reaction will definitely occur
- For example, a reaction may be feasible according to these rules, but have a very large activation energy barrier meaning that in reality it will not occur
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









