- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记6.2.8 Coloured Complexes
Coloured Compounds & Electron Promotion
- Most transition element complexes are coloured
- A transition element complex solution which is coloured, absorbs part of the electromagnetic spectrum in the visible light region
- The observed colour is the complementary colour which is made up of light with frequencies that are not absorbed
- For example, copper(II) ions absorb light from the red end of the spectrum
- The complementary colour observed is therefore pale blue (cyan)

The visible light region of the electromagnetic spectrum
Electron promotion
- In an isolated transition element ion (which is not bonded to any ligands), all of the 3d orbitals are degenerate
- However, when ligands are attached to the central metal ion through dative covalent bonds, these orbitals are split into two sets of non-degenerate orbitals
- The difference in energy between these two sets of orbitals is ΔE
- When light shines on a solution containing a transition element complex, an electron will absorb this exact amount of energy (ΔE)
- The amount of energy absorbed can be worked out by the equation:
ΔE = h x v
h = Planck's constant (6.626 x 10-34 m2 kg s-1)
v = frequency (Hertz, Hz or s-1)
- The electron uses the energy from the light to jump into a higher, non-degenerate energy level
- This is also called electron promotion
- The other frequencies of light which are not absorbed combine to make the complementary colour
- The diagram below shows an example of electron promotion in an octahedral complex of a nickel(II) Ni2+ ion
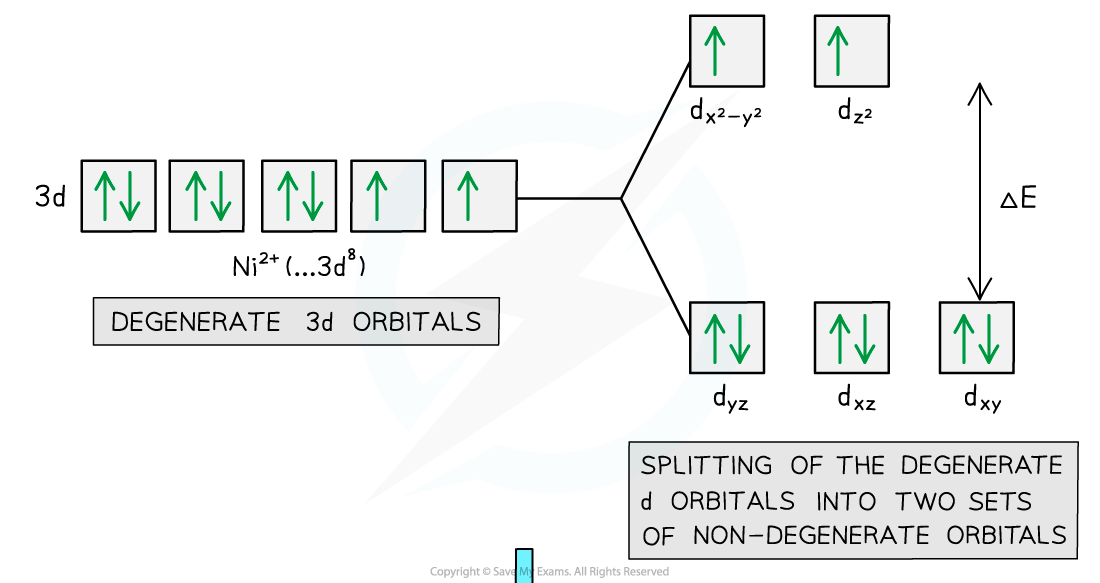
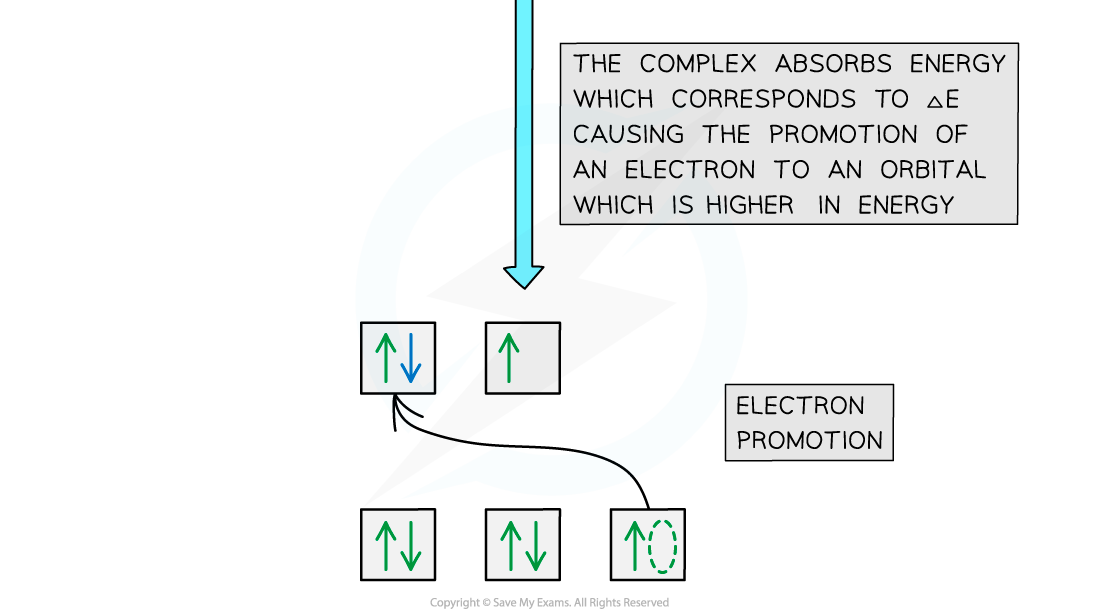
Electron promotion in a Ni(II) complex when light shines on the solution
Effects of Ligands on Complementary Colour
- Transition element complexes absorb the frequency of light which corresponds to the exact energy difference (ΔE) between their non-degenerate d orbitals
- The frequencies of light which are not absorbed combine to make the complementary colour of the complex
- It is the complementary colour which is seen
- However, the exact energy difference (ΔE) is affected by the different ligands which surround the transition element ion
- Different ligands will split the d orbital by a different amount of energy
- This depends on the repulsion that the d orbital experiences from these ligands
- Therefore, the size of ΔE and thus the frequency of light absorbed by the electrons will be slightly different
- As a result, a different colour of light is absorbed by the complex solution and a different complementary colour is observed
- This means that complexes with similar transition elements ions, but different ligands, can have different colours
- For example, the [Cu(H2O)6]2+ complex has a light blue colour
- Whereas the [Cu(NH3)4 (H2O)2]2+ has a dark blue colour
- Despite the copper ion having an oxidation state of +2 in both complexes
- This is evidence that the ligands surrounding the complex ion affect the colour of the complex
Ligand Exchange in Copper(II) & Cobalt(II) Complexes
- Different ligands may affect the complementary colour of a transition ion complex solution
- This is shown by ligand exchange reactions in copper(II) and cobalt(II) complexes, as this causes a change in colour of the complexes
Copper(II) & cobalt(II) ions
- The ligand exchange of [Cu(H2O)6]2+ and [Co(H2O)6]2+ by NH3 ligands causes a change in the colour of the solutions
- [Cu(H2O)6]2+ is light blue in colour whereas [Cu(NH3)4(H2O)2)]2+ is deep blue in colour
- [Co(H2O)6]2+ is a pink solution whereas [Co(NH3)6]2+ is a brown solution
- The colour change results from the ammonia ligands, which cause the d orbitals to split by a different amount of energy (ΔE)
- Therefore, the size of ΔE and the frequency of light absorbed by the electrons will be slightly different
- As a result, a different colour of light is absorbed and thus a different complementary colour is observed
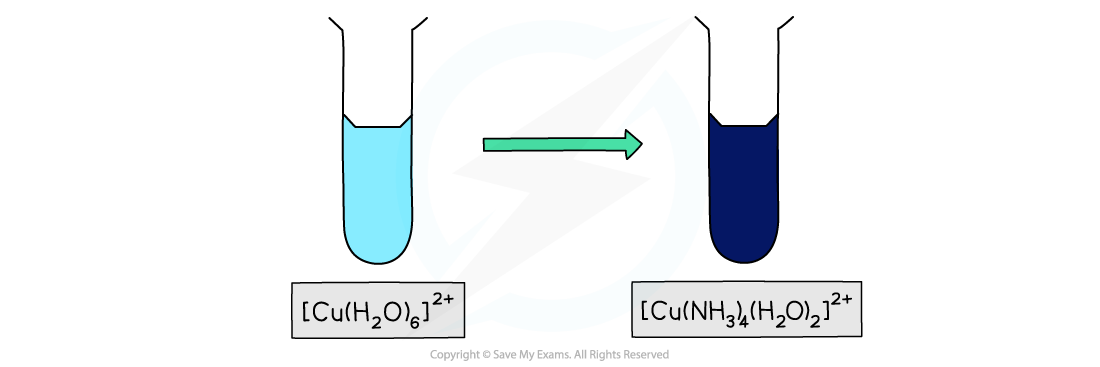
Ligand exchange of the water ligands by ammonia ligands causes a change in colour of the copper(II) complex solution
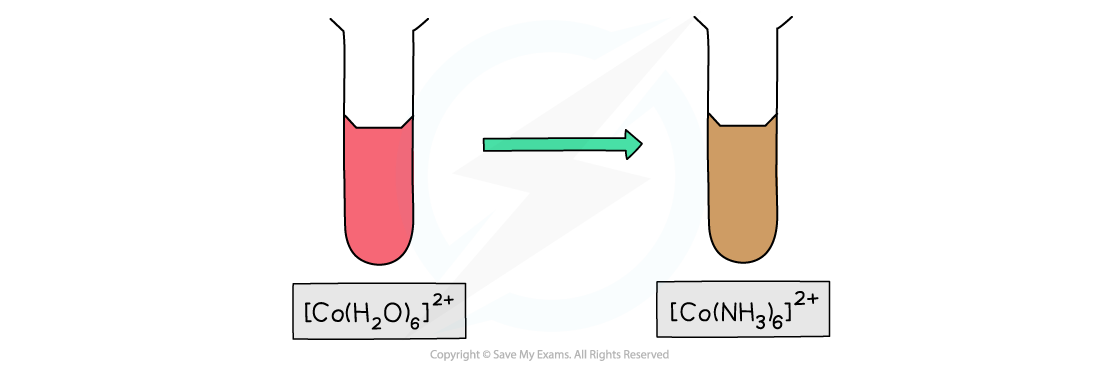
Ligand exchange of the water ligands by ammonia ligand causes a change in colour of the cobalt(II) complex solution
- Similarly, full ligand exchange by chloride ions in copper(II) and cobalt(II) complexes results in a change in complementary colour

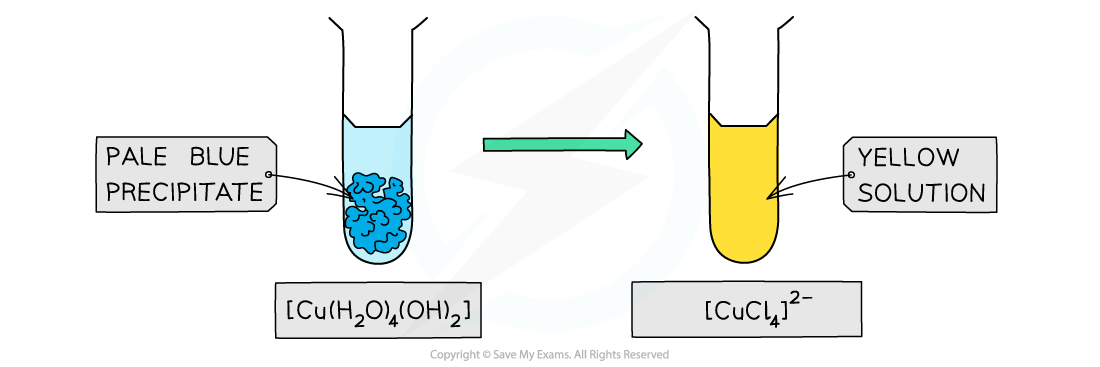
Ligand exchange by chloride ligands causes a change in colour of the copper(II) complex solution

Ligand exchange by chloride ligands causes a change in colour of the cobalt(II) complex solution
- As before, this suggests that different ligands will split the d orbitals differently
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









