- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记6.2.6 Geometry of Complexes
Geometry of the Transition Element Complexes
- Depending on the size of the ligands and the number of dative bonds to the central metal ion, transition element complexes have different geometries
- Dative bonds can also be referred to as coordinate bonds, especially when discussing the geometry of a complex
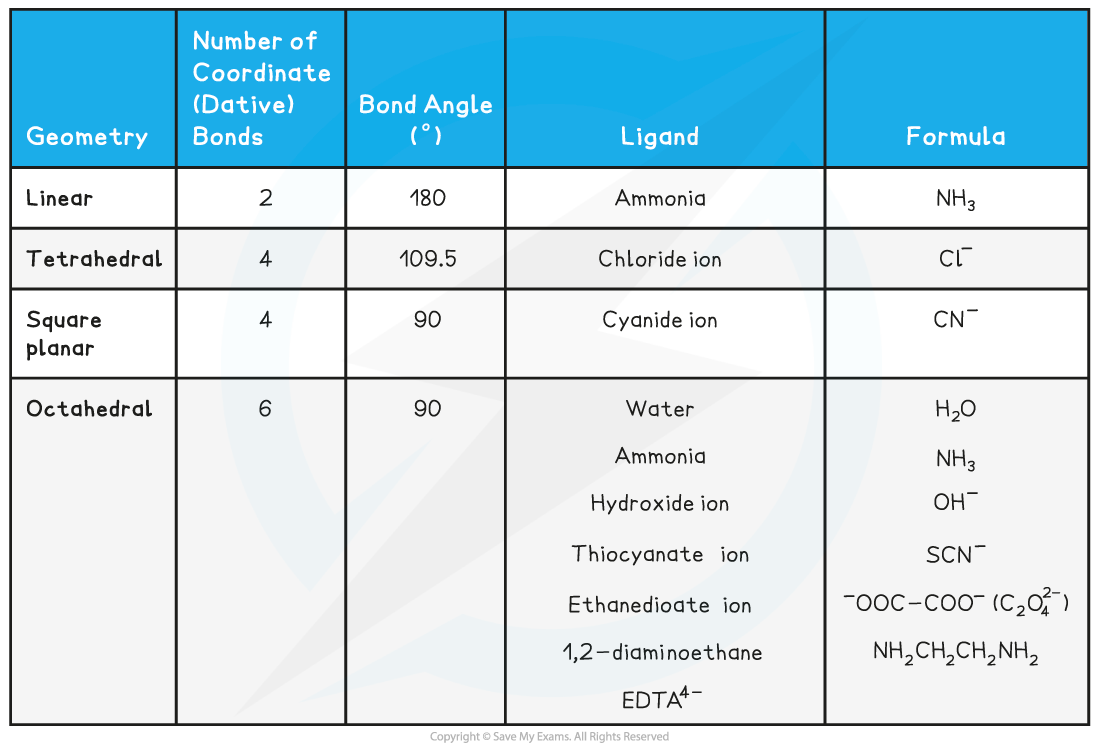
Linear
- Central metal atoms or ions with two coordinate bonds form linear complexes
- The bond angles in these complexes are 180o
- The most common examples are a copper (I) ion, (Cu+), or a silver (I) ion, (Ag+), as the central metal ion with two coordinate bonds formed to two ammonia ligands

Example of a linear complex
Tetrahedral
- When there are four coordinate bonds the complexes often have a tetrahedral shape
- Complexes with four chloride ions most commonly adopt this geometry
- Chloride ligands are large, so only four will fit around the central metal ion
- The bond angles in tetrahedral complexes are 109.5o

Example of a tetrahedral complex
Square planar
- Sometimes, complexes with four coordinate bonds may adopt a square planar geometry instead of a tetrahedral one
- Cyanide ions (CN-) are the most common ligands to adopt this geometry
- An example of a square planar complex is cisplatin
- The bond angles in a square planar complex are 90o

Cisplatin is an example of a square planar complex
Octahedral
- Octahedral complexes are formed when a central metal atom or ion forms six coordinate bonds
- This could be six coordinate bonds with six small, monodentate ligands
- Examples of such ligands are water and ammonia molecules and hydroxide and thiocyanate ions
- It could be six coordinate bonds with three bidentate ligands
- Each bidentate ligand will form two coordinate bonds, meaning six coordinate bonds in total
- Examples of these ligands are 1,2-diaminoethane and the ethanedioate ion
- It could be six coordinate bonds with one polydentate ligand
- The polydentate ligand, for example EDTA4-, forms all six coordinate bonds
- The bond angles in an octahedral complex are 90o
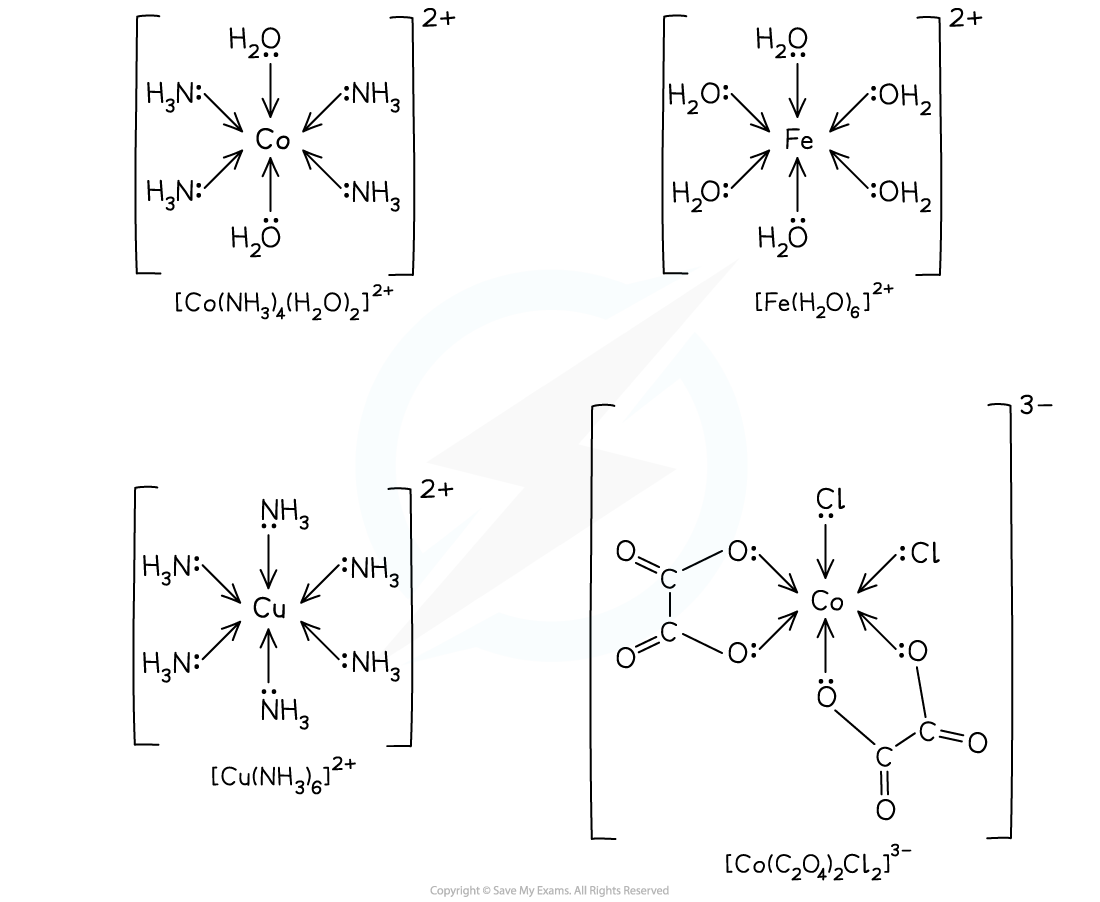
Examples of octahedral complexes
Types of ligands table
Coordination Number & Predicting Complex Ion Formula & Charge
- The coordination number of a complex is the number of coordinate bonds that are formed between the ligand(s) and the central metal atom or ion
- Some ligands can form only one coordinate bond with the central metal ion (monodentate ligands), whereas others can form two (bidentate ligands ) or more (polydentate ligands)
- It is not the number of ligands which determines the coordination number, it is the number of coordinate (dative) bonds
Predicting complex ion formula & charge
- The formula and charge of a complex ion can be predicted if the following are known:
- The central metal ion and its charge/oxidation state
- The ligands
- The coordination number/geometry
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









