- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记6.2.2 Oxidation States of Transition Metals
Effects of the 3d & 4s Subshells on Oxidation States of the Transition Elements
- Transition elements can have variable oxidation states
- These variable oxidation states can be formed as the 3d and 4s atomic orbitals are similar in energy
- This means that a similar amount of energy is needed to remove a different number of electrons
- When the transition elements form ions, the electrons of the 4s subshell are lost first, followed by the 3d electrons
- The most common oxidation state is +2, which is usually formed when the two 4s electrons are lost
Oxidation number at the start of the 3d transition elements
- At the start of the period, it is easier for the transition elements to lose the maximum number of electrons
- The maximum oxidation number of these transition elements involves all the 4s and 3d electrons in the atom
- For example, the maximum oxidation state of a titanium (Ti) ion is +3 or +4, as two 4s electrons and either 1 or 2 3d electrons are lost
- Ti atom = 1s2 2s2 2p6 3s2 3p6 3d2 4s2
- Ti3+ ion = 1s2 2s2 2p6 3s2 3p63d1
- Ti4+ ion = 1s2 2s2 2p6 3s2 3p6
Oxidation number at the end of the 3d transition elements
- Towards the end, the 3d transition elements are more likely to adopt the +2 oxidation state
- This is because across the d block, the 3d electrons become slightly harder to remove as the nuclear charge increases
- The 3d electrons are attracted more strongly to the nucleus
- The higher oxidation states become less stable
- Therefore, the elements are more likely to lose their 4s electrons only
- For example, nickel (Ni) is a transition element at the end of the period which only forms ions with oxidation state +2, due to loss of the 4s electrons only
- Ni atom = 1s2 2s2 2p6 3s2 3p6 3d8 4s2
- Ni2+ ion = 1s2 2s2 2p6 3s2 3p63d8
Transition Elements: Catalysts
- Transition elements are often used as catalysts due to their ability to form ions with more than one stable oxidation state, and the fact that they contain vacant d orbitals
Oxidation states
- Transition element ions can adopt more than one stable oxidation state
- This means that they can accept and lose electrons easily to go from one oxidation state to another
- They can therefore catalyse redox reactions, by acting as both oxidising agents and reducing agents
- For example, iron (Fe) is often used as a catalyst due to its ability to form Fe(II) and Fe(III) ions, acting as an oxidising agent and a reducing agent
- When Fe(II) acts as a reducing agent, it will reduce another species and become oxidised itself
Fe2+ → Fe3+ + e-
- The Fe3+ formed in the catalytic cycle, can then also act as an oxidising agent by oxidising another species and getting reduced itself to reform the Fe2+ ion
Fe3+ + e- → Fe2+
- Transition element ions with high oxidation states make powerful oxidising agents, because they will readily accept electrons
- A common example of this is potassium permanganate (VII), where manganese has an oxidation state of +7
Vacant d orbitals
- When transition elements form ions, they have vacant d orbitals which are energetically accessible
- The orbitals are not too high in energy
- This means that dative bonds can be formed between the transition element ion and ligands
- Each ligand provides the pair of electrons required for the formation of a bond between the ion and the ligand
- This pair of electrons is donated into the ion’s vacant d orbital
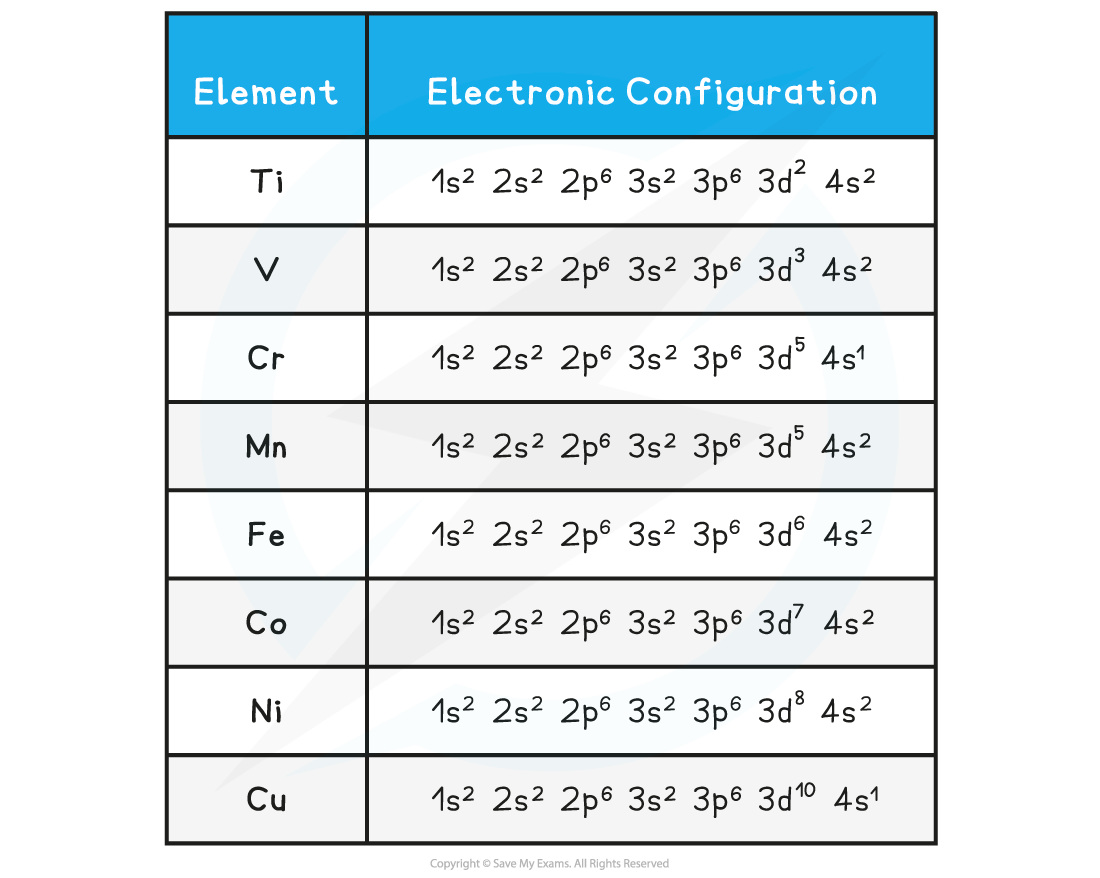
- The table below shows the electron configuration of the transition element atoms
- When they form ions, empty d orbitals are obtained which can be filled by the pairs of electrons donated by the ligands
Electronic configuration of transition elements table
Transition Metals: Complex Ions
- A complex is a molecule or ion formed by a central metal atom or ion surrounded by one or more ligands
- A complex can have an overall positive or negative charge, or it can be neutral
- If a complex is charged overall, it is often called a complex ion
- Transition elements can easily form complex ions, because they have empty d orbitals that are energetically accessible
- The empty d orbitals are therefore not too high in energy and can accommodate a lone pair of electrons
- The transition element in the centre will accept pairs of electrons from the ligands into their empty d orbitals, forming dative bonds
- The transition element in the centre is often referred to as the central metal ion, as all transition elements are metals, and it is often an ion in the centre
- For example, the titanium(III) (Ti3+) ion, has an electronic configuration of 1s2 2s2 2p6 3s2 3p63d1
- This means that there are vacant d orbitals that can be occupied by electrons, from ligands such as H2O for example, to form a [Ti(H2O)6]3+ complex ion
- 6 water ligands have each donated a pair of electrons, to form 6 dative bonds with the central metal ion
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








