- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记5.6.7 Homogeneous & Heterogeneous Catalysts
Homogeneous & Heterogeneous Catalysis
- Catalysts increase the rate of reaction by providing an alternative pathway which has a lower activation energy
- Catalysts can be either homogeneous or heterogeneous
- Homogeneous catalysts are those that are in the same phase as the reaction mixture
- For example, in the esterification of ethanoic acid (CH3COOH) with ethanol (CH3CH2OH) to form ethyl ethanoate (CH3COOCH2CH3) under acidic conditions
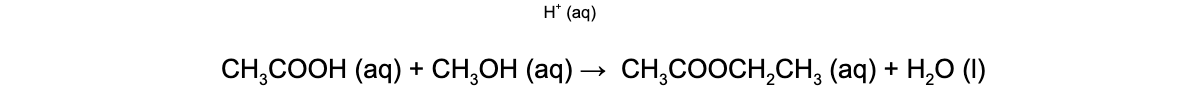
- The H+ is a homogeneous catalyst as like the reactants and product it is in the aqueous phase
- Heterogeneous catalysts are those that are in a different phase to the rest of the reaction mixture
- For example, in the Born-Haber process to form ammonia (NH3) from nitrogen (N2) and hydrogen (H2) an iron (Fe) catalyst is used
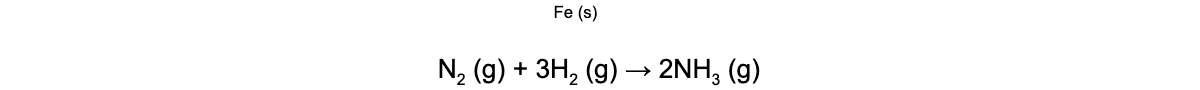
- The Fe catalyst is a heterogeneous catalyst as it is in the solid phase whereas the reactants and products are all in the gas phase
Heterogeneous Catalysis
- In heterogeneous catalysis, the molecules react at the surface of a solid catalyst
- The mode of action of a heterogeneous catalyst consists of the following steps:
- Adsorption (or chemisorption) of the reactants on the catalyst surface
- The reactants diffuse to the surface of the catalyst
- The reactant is physically adsorbed onto the surface by weak forces
- The reactant is chemically adsorbed onto the surface by stronger bonds
- Chemisorption causes bond weakening between the atoms of the reactants
- Desorption of the products
- The bonds between the products and catalyst weaken so much that the products break away from the surface
- For example, the adsorption of hydrogen molecules onto a palladium (Pd) surface
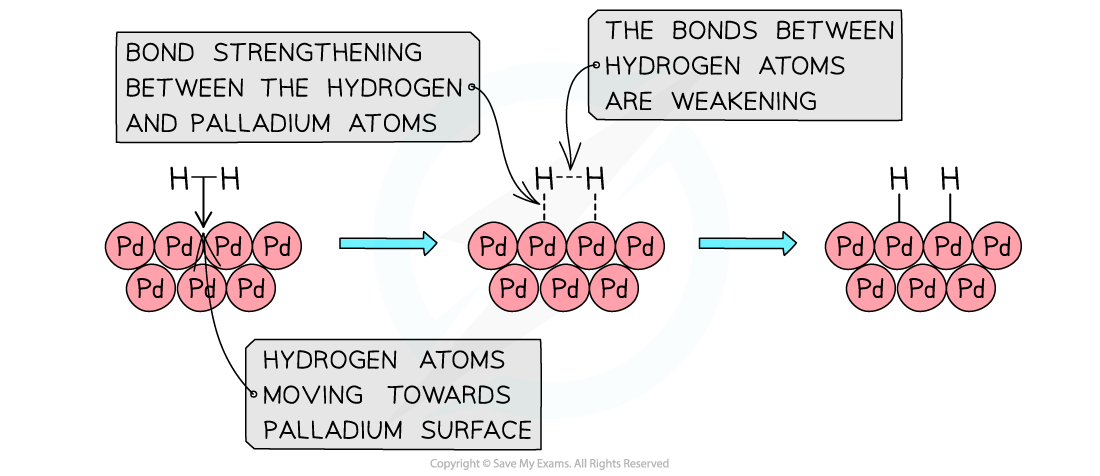
The reactants are adsorbed on the catalyst surface causing bond weakening and eventually desorption of the products
Iron in the Haber process
- In the Haber process ammonia (NH3) is produced from nitrogen (N2) and hydrogen (H2)
- An iron catalyst is used which speeds up the reaction by bringing the reactants close together on the metal surface
- This increases their likelihood to react with each other
- The mode of action of the iron catalyst is as follows:
- Diffusion of the nitrogen and hydrogen gas to the iron surface
- Adsorption of the reactant molecules onto the iron surface by forming bonds between the iron and reactant atoms
- These bonds are so strong that they weaken the covalent bonds between the nitrogen atoms in N2 and hydrogen atoms in H2
- But they are weak enough to break when the catalysis has been completed
- The reaction takes place between the adsorbed nitrogen and hydrogen atoms which react with each other on the iron surface to form NH3
- Desorption occurs when the bonds between the NH3 and iron surface are weakened and eventually broken
- The formed NH3 diffuses away from the iron surface
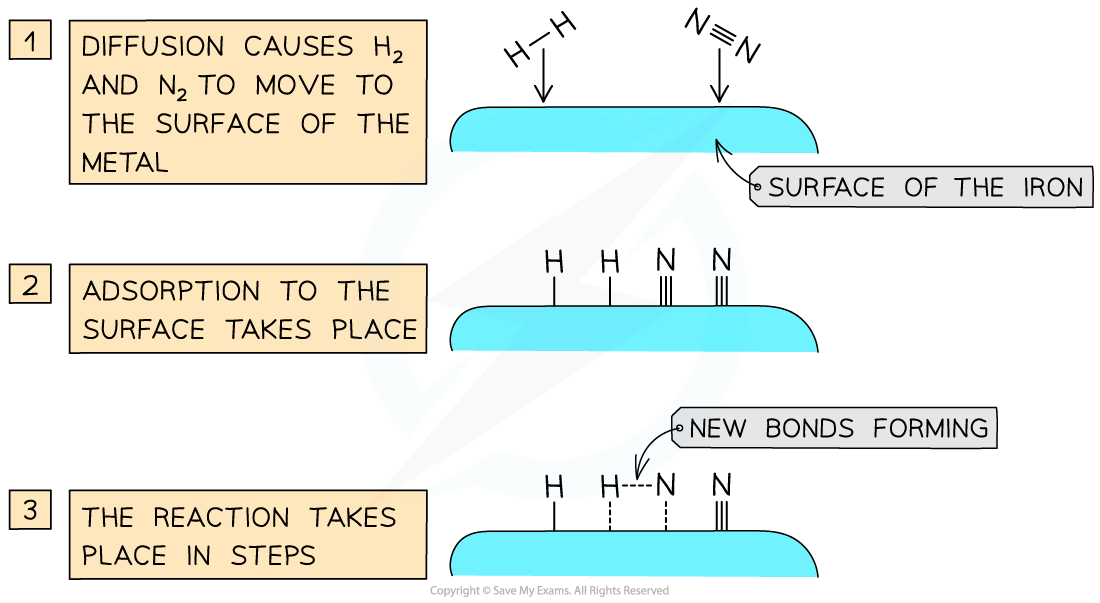
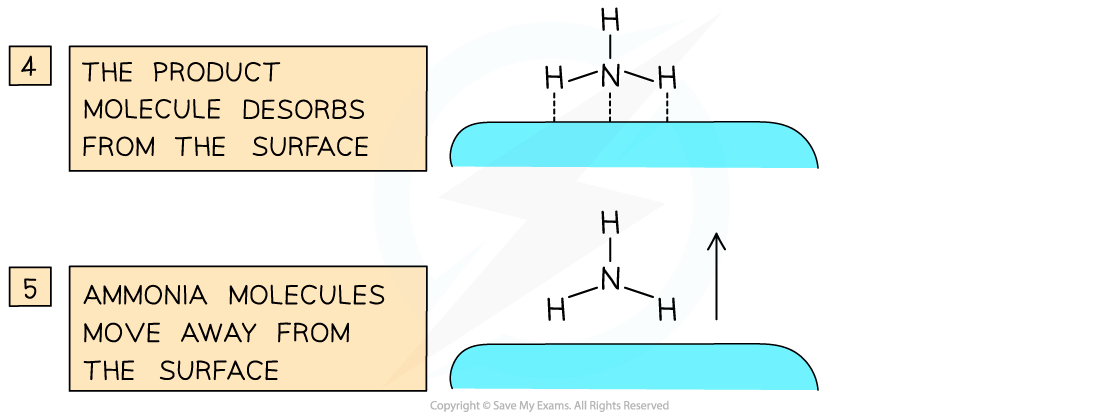
Iron brings the nitrogen and hydrogen closer together so that they can react and hence increases the rate of reaction
Heterogeneous catalyst in catalytic converters
- Heterogeneous catalysts are also used in the catalytic removal of oxides of nitrogen from the exhaust gases of car engines
- The catalysts speed up the conversion of:
- Nitrogen oxides (NOy) into harmless nitrogen gas (N2)
- Carbon monoxide (CO) into carbon dioxide (CO2)
- The catalytic converter has a honeycomb structure containing small beads coated with platinum, palladium, or rhodium metals which act as heterogeneous catalysts
- The mode of action of the catalysts is as following:
- Adsorption of the nitrogen oxides and CO onto the catalyst surface
- The weakening of the covalent bonds within nitrogen oxides and CO
- Formation of new bonds between:
- Adjacent nitrogen atoms to form N2 molecules
- CO and oxygen atoms to form CO2 molecules
- Desorption of N2 and CO2 molecules which eventually diffuse away from the metal surface
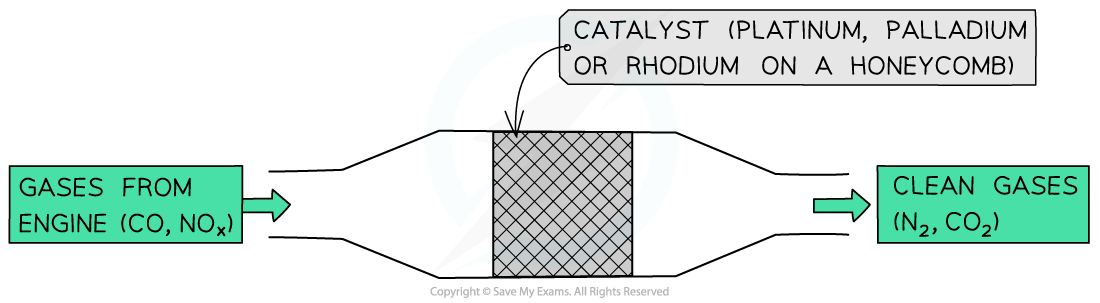
The metals in catalytic converters speed up the conversion of nitrogen oxides and CO into N2 and CO2 respectively
Homogeneous Catalysis
- Homogeneous catalysis often involves redox reactions in which the ions involved in catalysis undergo changes in their oxidation number
- As ions of transition metals can change oxidation number they are often good catalysts
- Homogeneous catalysts are used in one step and are reformed in a later step
The iodine-peroxydisulfate reaction
- This is a very slow reaction in which the peroxydisulfate (S2,O82- ) ions oxidise the iodide to iodine
S2O82- (aq) + 2I- (aq) → 2SO42- (aq) + I2 (aq)
- Since both the S2O82- and I- ions have a negative charge, it will require a lot of energy for the ions to overcome the repulsive forces and collide with each other
- Therefore, Fe3+ (aq) ions are used as a homogeneous catalyst
- The catalysis involves two redox reactions:
- First, Fe3+ ions are reduced to Fe2+ by I-
2Fe3+ (aq) + 2I- (aq) → 2Fe2+ (aq) + I2 (aq)
-
- Then, Fe2+ is oxidized back to Fe3+ by S2O82-
2Fe2+ (aq) + S2O82- (aq) → 2Fe3+ (aq) + 2SO42- (aq)
- By reacting the reactants with a positively charged Fe ion, there are no repulsive forces, and the activation energy is significantly lowered
- The order of the two reactions does not matter
- So, Fe2+ can be first oxidised to Fe3+ followed by the reduction of Fe3+ to Fe2+
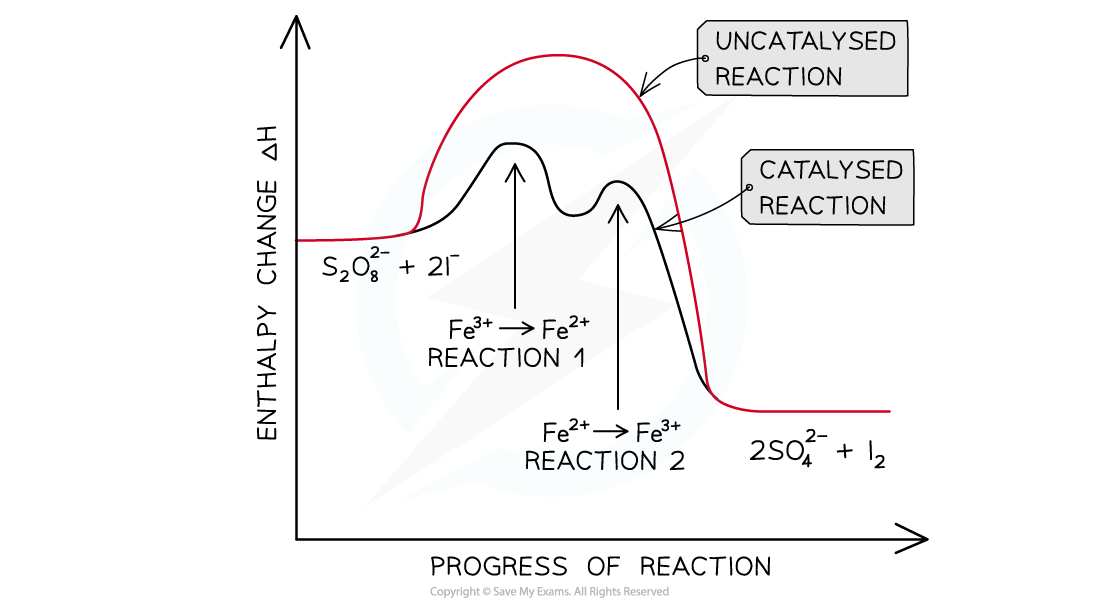
The catalysed reaction has two energy ‘humps’ because it is a two-stage reaction
Nitrogen oxides & acid rain
- As fossil fuels contain sulfur, burning the fuels will release sulfur dioxide which oxidises in air to sulfur trioxide, and then dilute sulfuric acid (H2SO4) is formed by reaction with water. The result is acidification of rain:
SO3(g) + H2O(l) → H2SO4(aq)
- Nitrogen oxides can act as catalysts in the formation of acid rain by catalysing the oxidation of SO2 to SO3
NO2(g) + SO2(g) → SO3(g) + NO(g)
- The formed NO gets oxidised to regenerate NO2
NO(g) + ½ O2(g) → NO2(g)
- The regenerated NO2 molecule can again oxidise another SO2 molecule to SO3 which will react with rainwater to form H2SO4 and so on
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1