- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记5.6.1 Basics of Kinetics
Kinetics: Basics
- The rate of reaction refers to the change in the amount or concentration of a reactant or product per unit time and can be found by:
- Measuring the decrease in the concentration of a reactant OR
- Measuring the increase in the concentration of a product over time
- The units of rate of reaction are mol dm-3 s-1
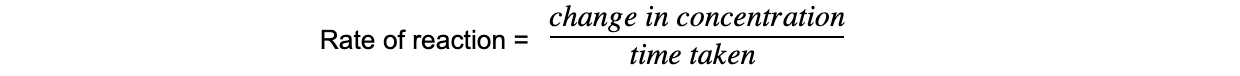
Rate equation
- The thermal decomposition of calcium carbonate (CaCO3) will be used as an example to study the rate of reaction
CaCO3 (s) → CaO (s) + CO2 (g)
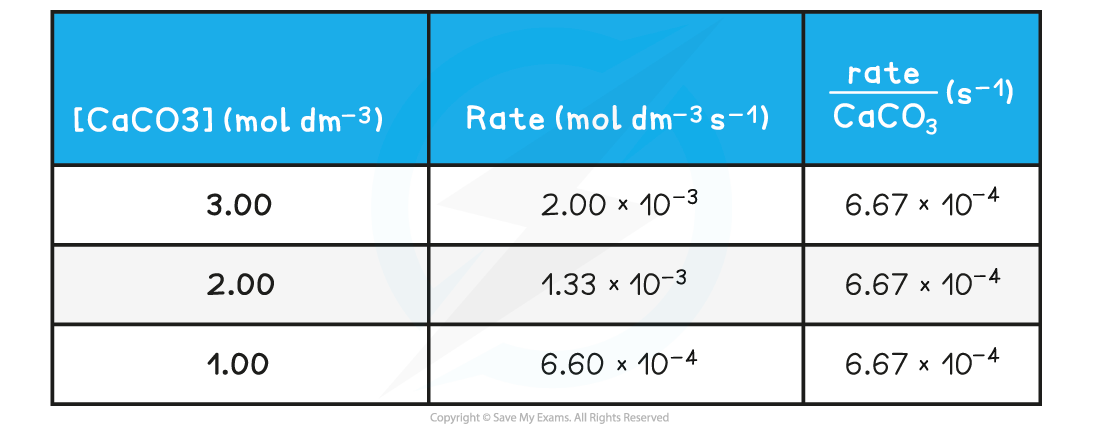
- The rate of reaction at different concentrations of CaCO3 is measured and tabulated

Rate of reactions table
- A directly proportional relationship between the rate of the reaction and concentration of CaCO3 is observed when a graph is plotted
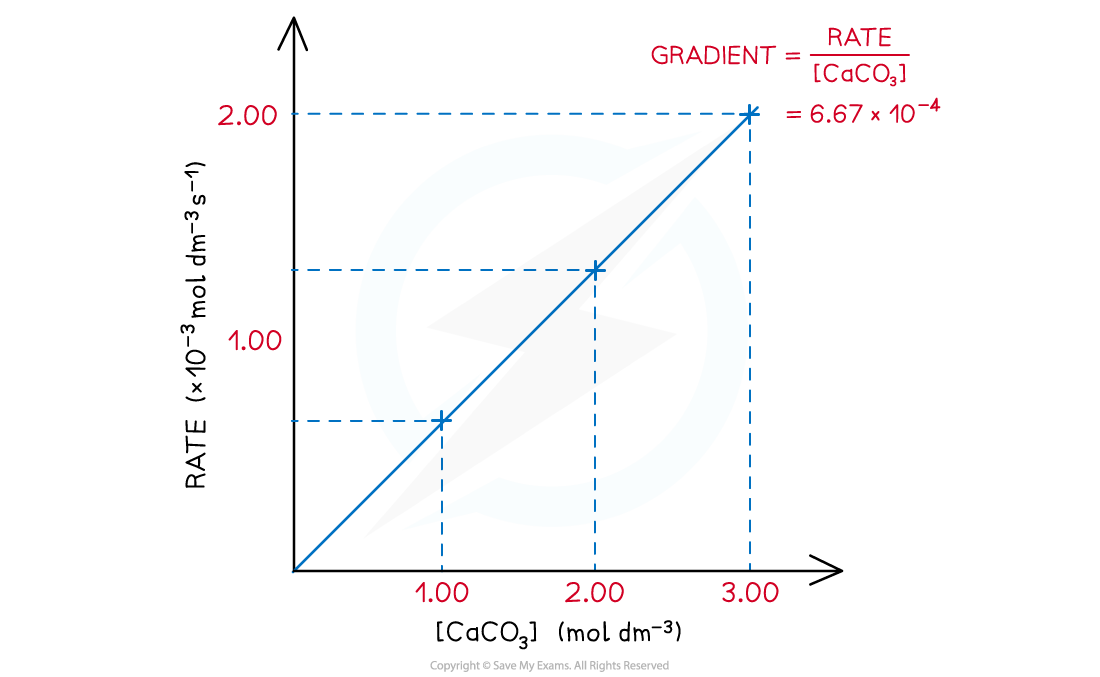
Rate of thermal decomposition of CaCO3 over the concentration of CaCO3
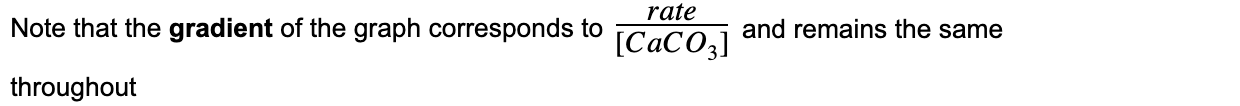
- The rate of reaction for the thermal decomposition of CaCO3 can also be written as:
Rate of reaction = k x [CaCO3]
- The proportionality constant k is the gradient of the graph and is also called the rate constant
- The rate equation is the overall expression for a particular reaction without the ‘x’ sign
Rate of reaction = k [CaCO3]
- Rate equations can only be determined experimentally and cannot be found from the stoichiometric equation
Rate of reaction = k [A]m [B]n
[A] and [B] = concentrations of reactants
m and n = orders of the reaction
- For example, the rate equation for the formation of nitrogen gas (N2) from nitrogen oxide (NO) and hydrogen (H2) is rate = k [NO]2 [H2]
2NO (g) + 2H2 (g) → N2 (g) + 2H2O (g)
rate = k [NO]2 [H2]
- As mentioned before, the rate equation of the reaction above cannot be deduced from the stoichiometric equation but can only experimentally be determined by:
- Changing the concentration of NO and determining how it affects the rate while keeping [H2] constant
- This shows that the rate is proportional to the square of [NO]
Rate = k1 [NO]2
- Then, changing the [H2] and determining how it affects the rate while keeping [NO] constant
- This shows that the rate is proportional to [H2]
Rate = k2 [H2]
- Combining the two equations gives the overall rate equation (where k = k1 + k2)
Rate = k [NO]2 [H2]
Order of reaction
- The order of reaction shows how the concentration of a reactant affects the rate of reaction
- It is the power to which the concentration of that reactant is raised in the rate equation
- The order of reaction can be 0, 1,2 or 3
- When the order of reaction of a reactant is 0, its concentration is ignored
- The overall order of reaction is the sum of the powers of the reactants in a rate equation
- For example, in the following rate equation, the reaction is:
Rate = k [NO2]2[H2]
-
- Second-order with respect to NO
- First-order with respect to H2
- Third-order overall (2 + 1)
Half-life
- The half-life (t1/2) is the time taken for the concentration of a limiting reactant to become half of its initial value
Rate-determining step & intermediates
- The rate-determining step is the slowest step in a reaction
- If a reactant appears in the rate-determining step, then the concentration of that reactant will also appear in the rate equation
- For example, the rate equation for the reaction below is rate = k [CH3Br] [OH-]
CH3Br + OH- → CH3OH + Br-
-
- This suggests that both CH3Br and OH- take part in the slow rate-determining step
- This reaction is, therefore, a bimolecular reaction
- Unimolecular: one species involved in the rate-determining step
- Bimolecular: two species involved in the rate-determining step
- The intermediate is derived from substances that react together to form it in the rate-determining step
- For example, for the reaction above the intermediate would consist of CH3Br and OH-

The intermediate is formed from the species that are involved in the rate-determining step (and thus appear in the rate equation)
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








