- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记5.5.9 Partition Coefficients
Partition Coefficient & Calculations
- The partition coefficient (Kpc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact with each other when equilibrium has been established (at a particular temperature)
- For example, methylamine (CH3NH2) is dissolved in two immiscible solvents:
- Water
- An organic solvent
- A separating funnel is shaken with the organic solvent and aqueous methylamine
- The methylamine is soluble in both solvents, so when the mixture is left to settle an equilibrium is established
- The rate of methylamine molecules moving from the organic layer into the aqueous layer is equal to the rate of molecules moving from the aqueous layer to the organic layer
CH3NH2(aq) ⇌ CH3NH2(organic solvent)
- The value of its equilibrium constant is also called the partition coefficient
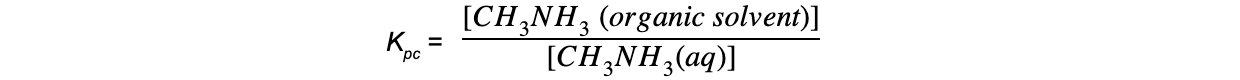
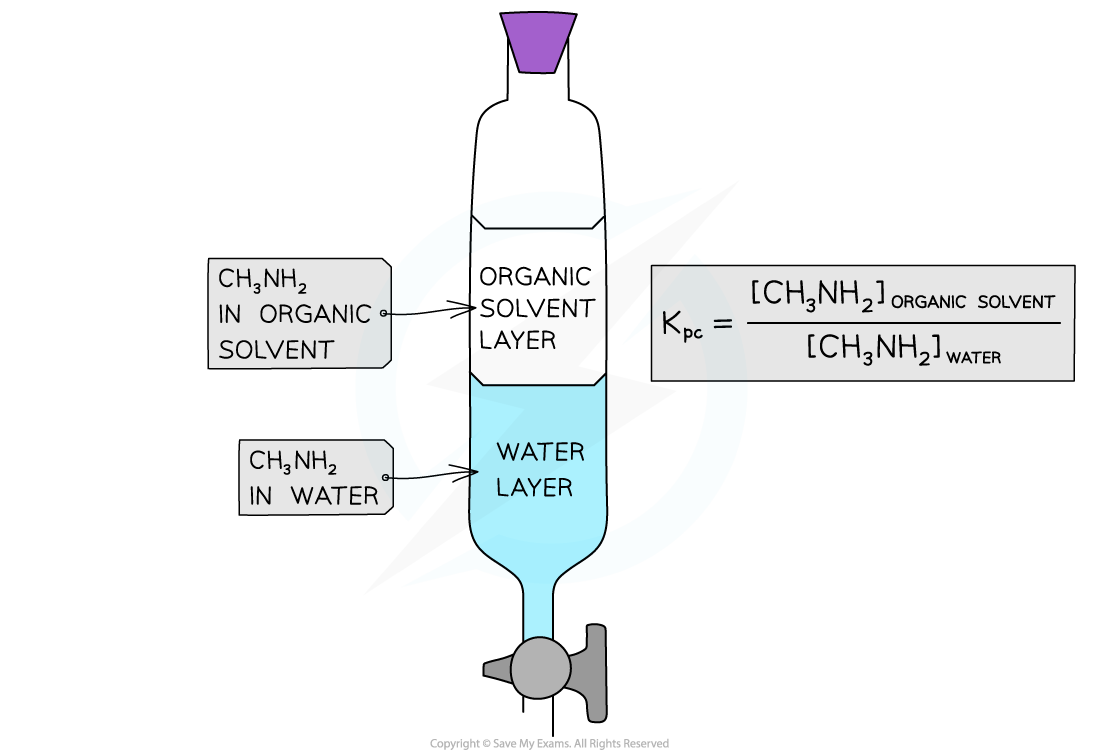
The partition coefficient is the ratio of methylamine molecules in the organic and aqueous layer once equilibrium has been established
Calculating Partition Coefficients
- The partition coefficient (Kpc) for a system in which the solute is in the same physical state in the two solvents can be calculated using the equilibrium expression
Worked example: Calculating the partition coefficient
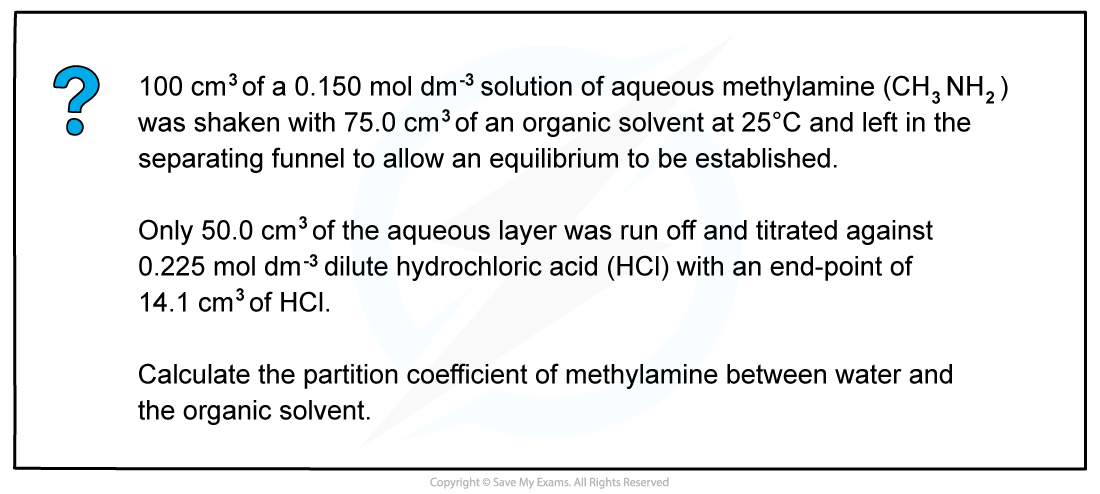
Answer
- Step 1: Write down the equilibrium equation
CH3NH2(aq) ⇌ CH3NH2(organic solvent)
- Step 2: Write down the equilibrium expression
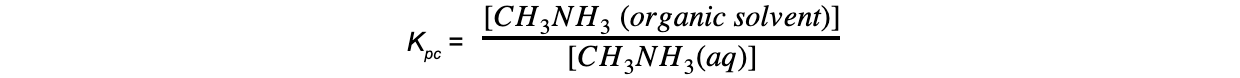
- Step 3: Determine how many moles of CH3NH2 has reacted with HCl at the end-point
At the end-point, all CH3NH2 (aq) has been neutralised by HCl (aq)
CH3NH2 (aq) + HCl (aq) → CH3NH3Cl (aq)
CH3NH2 and HCl react in a ratio of 1:1
Mol (HCl) = mol (CH3NH2) = 0.225 x 0.0141
= 3.18 x 10-3 mol
- Step 4: Determine the number of moles of CH3NH2 present in the aqueous layer
Only 50.0 cm3 of the aqueous layer was used to titrate against HCl
Thus, 3.18 x 10-3 mol of CH3NH2 was present in only 50.0 cm3 of the aqueous layer
The number of moles of CH3NH2 in 100 cm3 aqueous layer is, therefore:
Mol (CH3NH2 aqueous layer) = 3.18 x 10-3 x 2 = 6.34 x 10-3 mol
- Step 5: Determine the number of moles of CH3NH2 in the organic layer
Mol CH3NH2 (organic layer) = mol CH3NH2 (total) - mol CH3NH2(aqueous layer)
Mol CH3NH2 (total) = 0.100 x 0.150 = 0.015 mol
Mol CH3NH2 (organic layer) = 0.015 - 6.34 x 10-3 = 8.67 x 10-3 mol
- Step 6: Change the number of moles into concentrations

= 0.063 mol dm-3

= 0.116 mol dm-3
- Step 7: Substitute the values into the Kpc expression
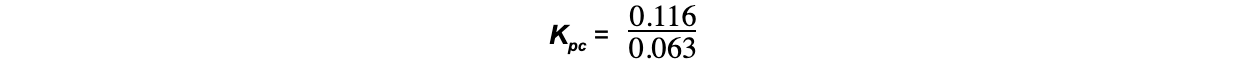
= 1.83
Since the value of Kpc is larger than 1, methylamine is more soluble in the organic solvent than in water
Factors Affecting the Partition Coefficient
- The partition coefficient (Kpc) depends on the solubilities of the solute in the two solvents
- The degree of solubility of a solute is determined by how strong the intermolecular bonds between solute and solvent are
- The strength of these intermolecular bonds, in turn, depends on the polarity of the solute and solvent molecules
- For example, ammonia is more soluble in water than in an organic solvent such as carbon tetrachloride (CCl4)
- Ammonia and water are both polar molecules that form hydrogen bonds with each other
- Ammonia forms permanent dipole-induced dipole forces with the non-polar CCl4 molecules
- Since these forces are much weaker than hydrogen bonding, ammonia is less soluble in CCl4
- When Kpc is < 1 the solute is more soluble in water than the organic solvent
- When Kpc is > 1 the solute is more soluble in the organic solvent than the water
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








