- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记5.5.8 The Common Ion Effect
Solubility Product & the Common Ion Effect
- A saturated solution is a solution that contains the maximum amount of dissolved salt
- If a second compound, which has an ion in common with the dissolved salt, is added to the saturated solution, the solubility of the salt reduces and a solid precipitate will be formed
- This is also known as the common ion effect
- For example, if a solution of potassium chloride (KCl) is added to a saturated solution of silver chloride (AgCl) a precipitate of silver chloride will be formed
- The chloride ion is the common ion
- The solubility product can be used to predict whether a precipitate will actually form or not
- A precipitate will form if the product of the ion concentrations is greater than the solubility product (Ksp)
Common ion effect in silver chloride
- When a KCl solution is added to a saturated solution of AgCl, an AgCl precipitate forms
- In a saturated AgCl solution, the silver chloride is in equilibrium with its ions
AgCl (s) ⇌ Ag+ (aq) + Cl- (aq)
- When a solution of potassium chloride is added:
- Both KCl and AgCl have the common Cl- ion
- There is an increased Cl- concentration so the equilibrium position shifts to the left
- The increase in Cl- concentration also means that [Ag+ (aq)] [Cl-(aq)] is greater than the Ksp for AgCl
- As a result, the AgCl is precipitated
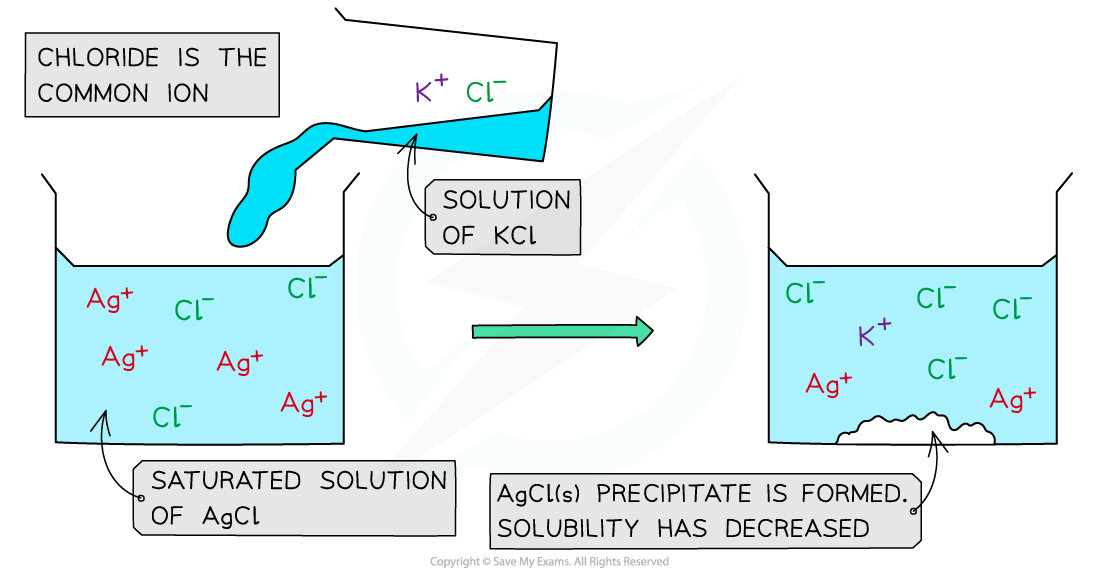
The addition of potassium chloride to a saturated solution of silver chloride results in the precipitate of silver chloride
Worked Example: Calculations using the Ksp values and the concentration of the common ion

Answer
- Step 1: Determine the equilibrium reaction of CaSO4
CaSO4 (s) ⇌ Ca2+ (aq) + SO42- (aq)
- Step 2: Write down the equilibrium expression for Ksp
Ksp = [Ca2+ (aq)] [SO42- (aq)]
- Step 3: Determine the concentrations of the ions
There are equal volumes of each solution
This means that the total solution was diluted by a factor of 2
The new concentrations of the Ca2+ ion is halved
![]() = 5.0 x 10-4 mol dm-3
= 5.0 x 10-4 mol dm-3
The sulfate ion concentration remains the same as it is a common ion and its concentration is the same in both solutions
- Step 4: Substitute the values into the expression
Product of the ion concentrations = [Ca2+ (aq)] x [SO42- (aq)]
= (5.0 x 10-4) x (1.0 x 10-3)
= 5.0 x 10-7 mol2 dm-6
- Step 5: Determine if a precipitate will form
As the product of the ion concentration (5.0 x 10-7 mol dm-3 ) is smaller than the Ksp value (2.0 x 10-5 mol2 dm-6), the CaSO4 precipitate will not be formed
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








