- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记5.5.6 Solubility Product
Solubility Product
- Solubility is defined as the number of grams or moles of compound needed to saturate 100 g of water, or it can also be defined in terms of 1 kg of water, at a given temperature
- For example, sodium chloride (NaCl) is considered to be a soluble salt as a saturated solution contains 36 g of NaCl per 100 g of water
- Lead chloride (PbCl2) on the other hand is an insoluble salt as a saturated solution only contains 0.99 g of PbCl2 per 100 g of water
Solubility product
- The solubility product (Ksp) is:
- The product of the concentrations of each ion in a saturated solution of a relatively soluble salt
- At 298 K
- Raised to the power of their relative concentrations
C (s) ⇌ aAx+ (aq) + bBy- (aq)
Ksp = [Ax+ (aq)]a [By- (aq)]b
- When an undissolved ionic compound is in contact with a saturated solution of its ions, an equilibrium is established
- The ions move from the solid to the saturated solution at the same rate as they move from the solution to the solid
- For example, the undissolved magnesium chloride (MgCl2) is in equilibrium with a saturated solution of its ions
MgCl2 (s) ⇌ Mg2+ (aq) + 2Cl- (aq)
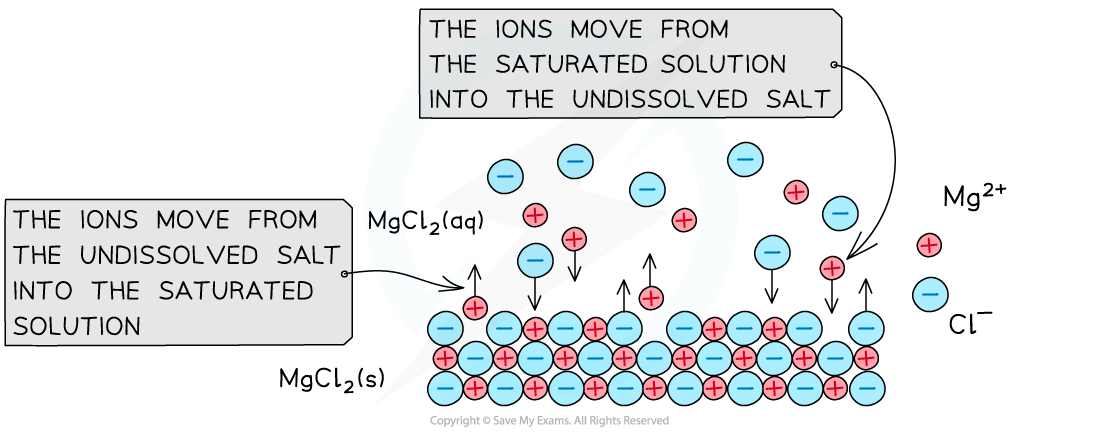
When the undissolved MgCl2 salt gets in contact with its ions in a saturated solution, an equilibrium between the salt and ions is established
-
- The solubility product for this equilibrium is:
Ksp = [Mg2+ (aq)] [Cl- (aq)]2
- The Ksp is only useful for sparingly soluble salts
- The smaller the value of Ksp, the lower the solubility of the salt
Expressing Ksp
- The general equilibrium expression for the solubility product (Ksp) is:
C (s) ⇌ aAx+ (aq) + bBy- (aq)
Ksp= [Ax+ (aq)]a [By- (aq)]b
Worked Example: Expressing Ksp of ionic compounds
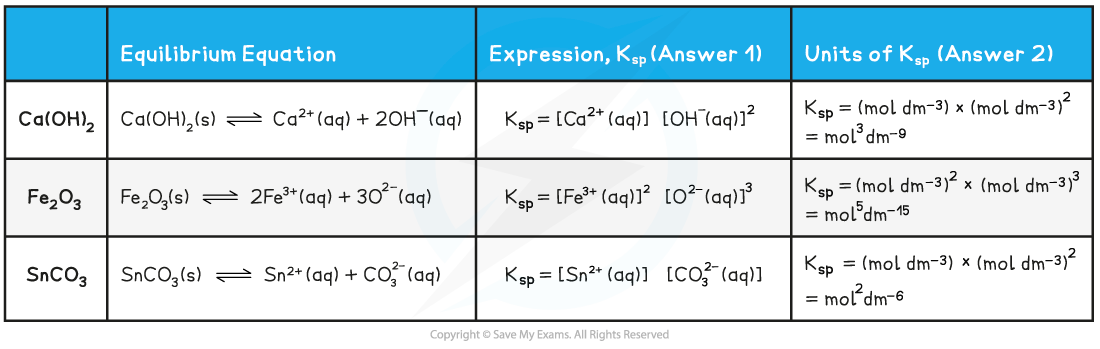
Answer
Expressing Ksp of ionic compounds answers table
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








