- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记5.5.3 pH & [H+] Calculations
Calculating [H+] & pH
- If the concentration of H+ of an acid or alkali is known, the pH can be calculated using the equation:
pH = -log [H+]
- Similarly, the concentration of H+ of a solution can be calculated if the pH is known by rearranging the above equation to:
[H+] = 10-pH
Strong acids
- Strong acids are completely ionised in solution
HA (aq) → H+ (aq) + A- (aq)
- Therefore, the concentration of hydrogen ions ([H+]) is equal to the concentration of acid ([HA])
- The number of hydrogen ions ([H+]) formed from the ionisation of water is very small relative to the [H+] due to ionisation of the strong acid and can therefore be neglected
- The total [H+] is therefore the same as the [HA]
Worked Example: pH calculations of a strong acid
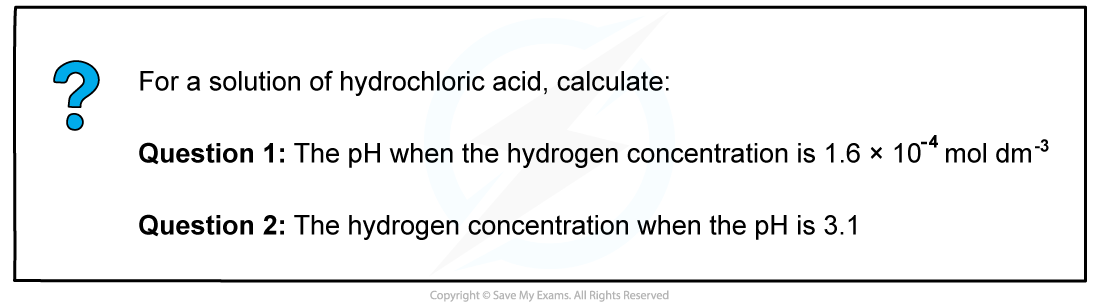
Answer
Hydrochloric acid is a strong monobasic acid
HCl (aq) → H+ (aq) + Cl- (aq)
Answer 1
The pH of the solution is:pH = -log [H+]
= -log 1.6 x 10-4
= 3.80
Answer 2
The hydrogen concentration can be calculated by rearranging the equation for pH
pH = -log [H+]
[H+] = 10-pH
= 10-3.1
= 7.9 x 10-4 mol dm-3
Strong alkalis
- Strong alkalis are completely ionised in solution
BOH (aq) → B+ (aq) + OH- (aq)
- Therefore, the concentration of hydroxide ions ([OH-]) is equal to the concentration of base ([BOH])
- Even strong alkalis have small amounts of H+ in solution which is due to the ionisation of water
- The concentration of OH- in solution can be used to calculate the pH using the ionic product of water
Kw = [H+] [OH-]
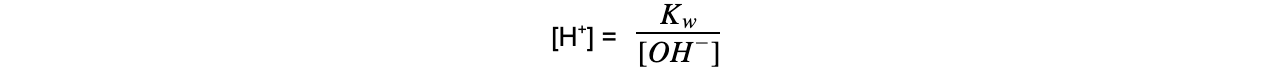
- Since Kw is 1.00 x 10-14 mol2 dm-6
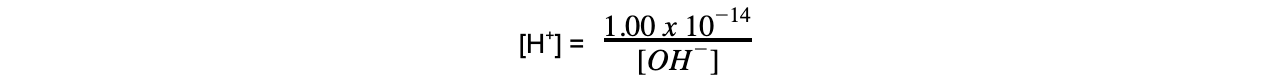
- Once the [H+] has been determined, the pH of the strong alkali can be founding using pH = -log[H+]
- Similarly, the ionic product of water can be used to find the concentration of OH- ions in solution if [H+] is known
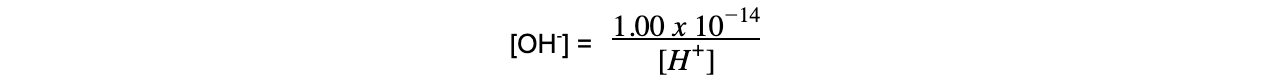
Worked Example: pH calculations of a strong alkali
Answer
Sodium hydroxide is a strong base which ionises as follows:
NaOH (aq) → Na+ (aq) + OH- (aq)
Answer 1
The pH of the solution is:pH = -log [H+]
= -log 3.5 x 10-11
= 10.5
Answer 2
- Step 1: Calculate hydrogen concentration by rearranging the equation for pH
pH = -log [H+]
= 10-pH
= 10-12.3
= 5.01 x 10-13 mol dm-3
- Step 2: Rearrange the ionic product of water to find the concentration of hydroxide ions
Kw = [H+] [OH-]
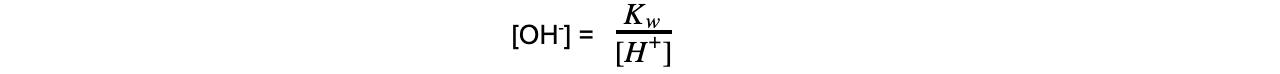
- Step 3: Substitute the values into the expression to find the concentration of hydroxide ions
Since Kw is 1.00 x 10-14 mol2 dm-6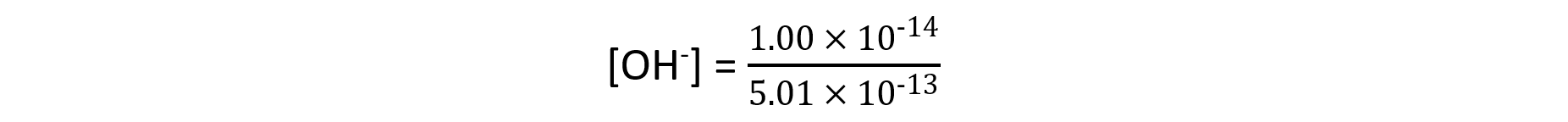
= 0.0199 mol dm-3
Weak acids
- The pH of weak acids can be calculated when the following is known:
- The concentration of the acid
- The Ka value of the acid
Worked Example: pH calculations of weak acids

Answer
Ethanoic acid is a weak acid which ionises as follows:
CH3COOH (aq) ⇌ H+ (aq) + CH3COO- (aq)
- Step 1: Write down the equilibrium expression to find Ka
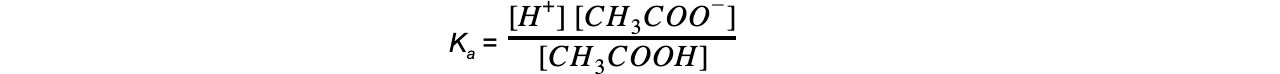
- Step 2: Simplify the expression
The ratio of H+ to CH3COO- ions is 1:1
The concentration of H+ and CH3COO- ions are therefore the same
The expression can be simplified to:
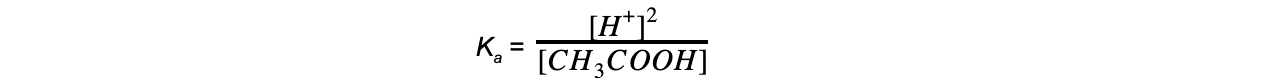
- Step 3: Rearrange the expression to find [H+]
![]()
- Step 4: Substitute the values into the expression to find [H+]
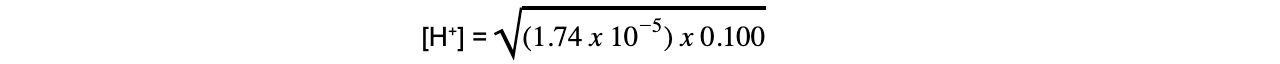 = 1.32 x 10-3 mol dm-3
= 1.32 x 10-3 mol dm-3
- Step 5: Find the pH
pH = -log10 [H+]
= -log10 1.32 x 10-3
= 2.88
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









