- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记5.4.6 Standard Electrode Potentials: Free Energy Change
Calculating Free Energy Change Using Standard Electrode Potentials
- The standard free energy change can be calculated using the standard cell potential of an electrochemical cell
ΔGꝋ = - n x Ecellꝋ x F
ΔGꝋ = standard Gibbs free energy
n = number of electrons transferred in the reaction
Ecellꝋ = standard cell potential (V)
F = Faraday constant (96 500 C mol-1)
Worked Example: Calculating the standard Gibbs free energy change
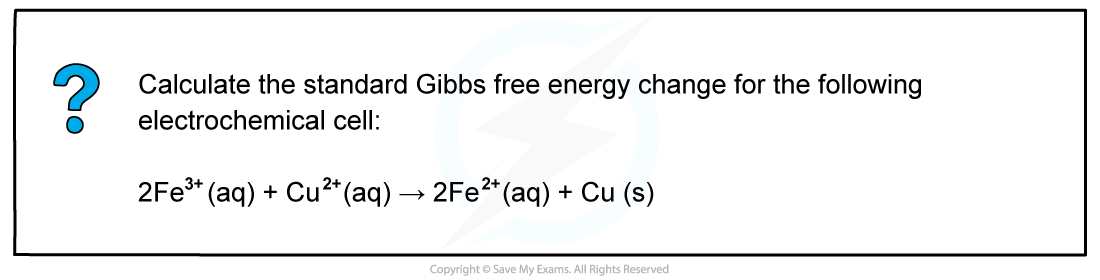
Answer
- Step 1: Determine the two half-equations and their Eꝋ using the Data booklet
Fe3+ (aq) + e- ⇌ Fe2+ (aq) Eꝋ = +0.77 V
Cu2+ (aq) + 2e- ⇌ Cu (s) Eꝋ = +0.34 V
- Step 2 : Calculate the Ecellꝋ
Ecellꝋ = Eredꝋ - Eoxꝋ
= (+0.77) - (+0.34)
= +0.43 V
- Step 3: Determine the number of electrons transferred in the reaction
The Cu2+/Cu has a smaller Eꝋ value which means that it gets oxidised
It transfers two electrons to two Fe3+ ions
Each Fe3+ ion accepts one electron so the total number of electrons transferred is two
- Step 4: Substitute the values in for the standard Gibbs free energy equation
ΔGꝋ = - n x Ecellꝋ x F
= -2 x (+0.43) x 96 500
= -82 990 J mol-1
= -83 kJ mol-1
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









