- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记5.4.5 Nernst Equation
The Nernst Equation
- Under non-standard conditions, the cell potential of the half-cells is shown by the symbol Ecell
- The effect of changes in temperature and ion concentration on the Ecell can be deduced using the Nernst equation
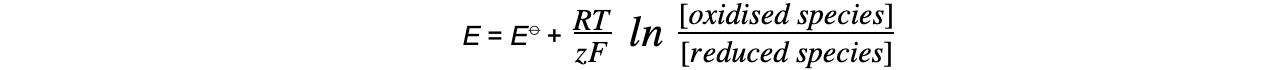
E = electrode potential under nonstandard conditions
E⦵ = standard electrode potential
R = gas constant (8.31 J K-1 mol-1)
T = temperature (kelvin, K)
z = number of electrons transferred in the reaction
F = Faraday constant (96 500 C mol-1)
ln = natural logarithm
- This equation can be simplified to
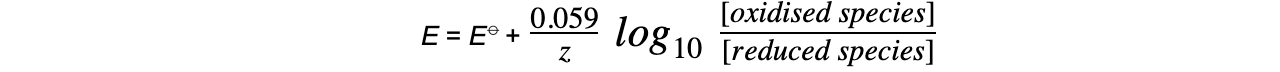
-
- At standard temperature, R, T and F are constant
- ln x = 2.303 log10 x
- The Nernst equation only depends on aqueous ions and not solids or gases
- The concentrations of solids and gases are therefore set to 1.0 mol dm-3
Applying Nernst equation
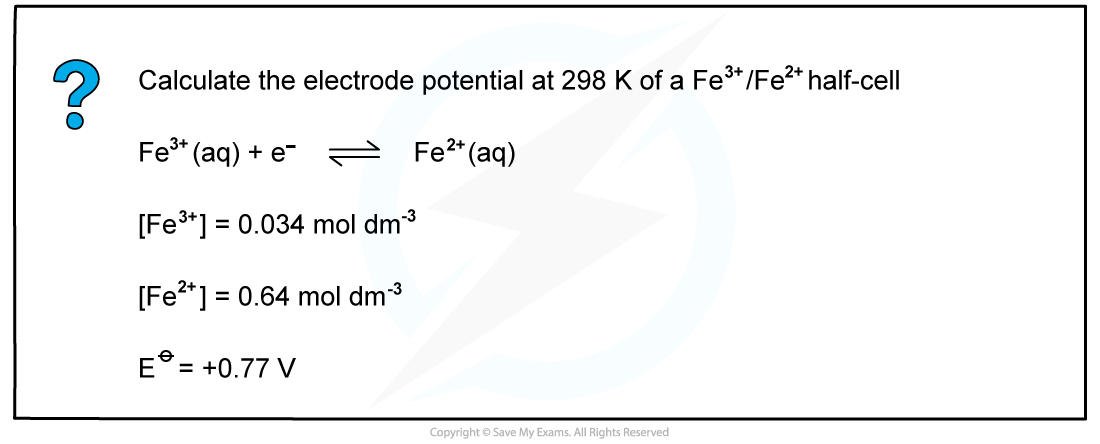
- The concentrations of ions for the Fe3+/Fe2+ half-cell are as follows:
Fe3+ (aq) + e- ⇌ Fe2+ (aq)
[Fe3+] = 0.034 mol dm-3
[Fe2+] = 0.64 mol dm-3
- The Nernst equation for this half-reaction is, therefore:
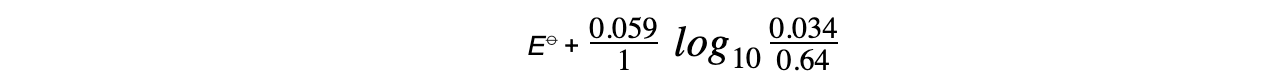
-
- The oxidised species is Fe3+ as it has a higher oxidation number (+3)
- The reduced species is Fe2+ as it has a lower oxidation number (+2)
- z is 1 as only one electron is transferred in this reaction
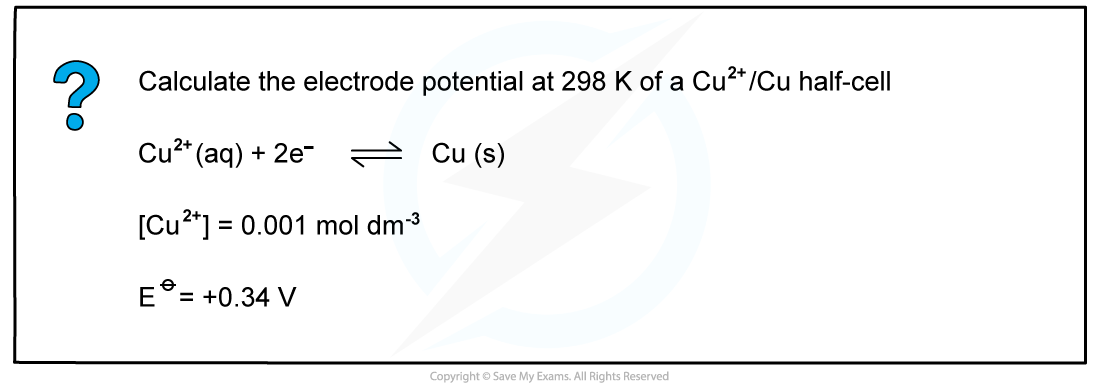
- An example of a half-cell in which two electrons are transferred is the Cu2+/Cu half-cell
Cu2+ (aq) + 2e- ⇌ Cu (s)
[Cu2+] = 0.0010 mol dm-3
- The Nernst equation for this half-reaction is:
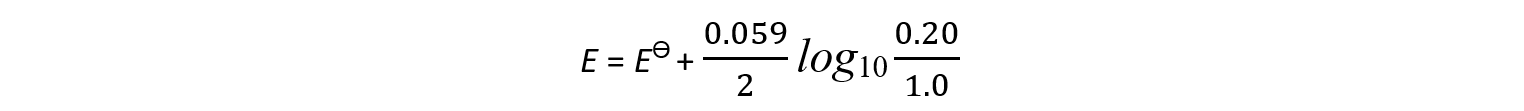
-
- The oxidised species is Cu2+ as it has a higher oxidation number (+2)
- The reduced species is Cu as it has a lower oxidation number (0)
- Cu is a solid and is not included in the Nernst equation (its concentration doesn’t change)
- z is 2 as 2 electrons are transferred in this reaction
Worked example: Calculating the electrode potential of a Fe3+/Fe2+ half-cell
Answer
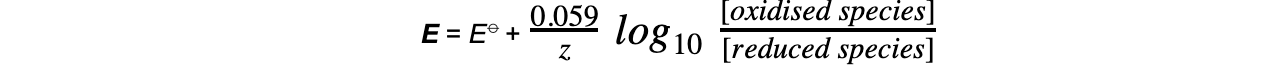
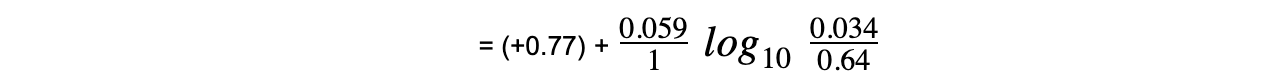
= (+0.77) + (-0.075)
= +0.69 V
Worked example: Calculating the electrode potential of a Cu2+/Cu half-cell
Answer
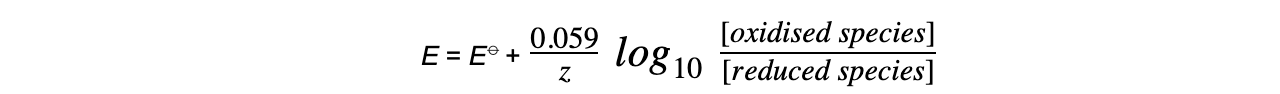
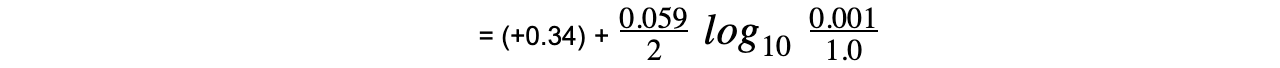
= (+0.34) + (-0.089)
= +0.25 V
Exam Tip
Make sure you always check what the temperature is. If the temperature is not 298 K (or 25 oC) the full Nernst equation should be used.You don’t need to know how to simplify the Nernst equation to
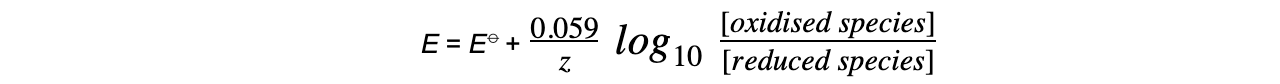
You are only expected to use the equation when the temperature is 298 K (or 25 oC).


最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








