- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记5.4.2 Standard Cell Potential: Calculations, Electron Flow & Feasibility
Calculating Standard Cell Potential
- Once the standard electrode potentials (Eꝋ) of the half-cells are determined, the standard cell potential (Ecellꝋ) can be calculated by subtracting the less positive Eꝋ from the more positive Eꝋ value
- The half-cell with the more positive Eꝋ value will be the positive pole
- The half-cell with the less positive Eꝋ value will be the negative pole
Worked example: Calculating the standard cell potential
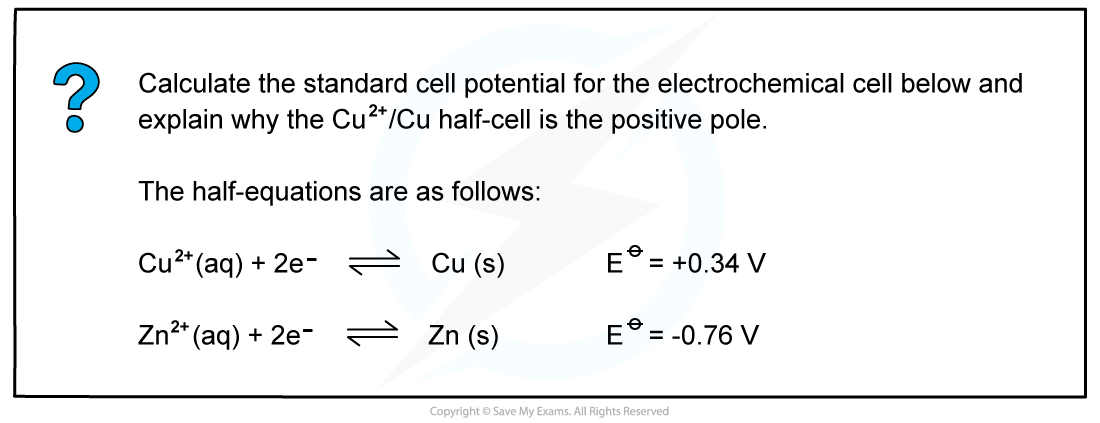
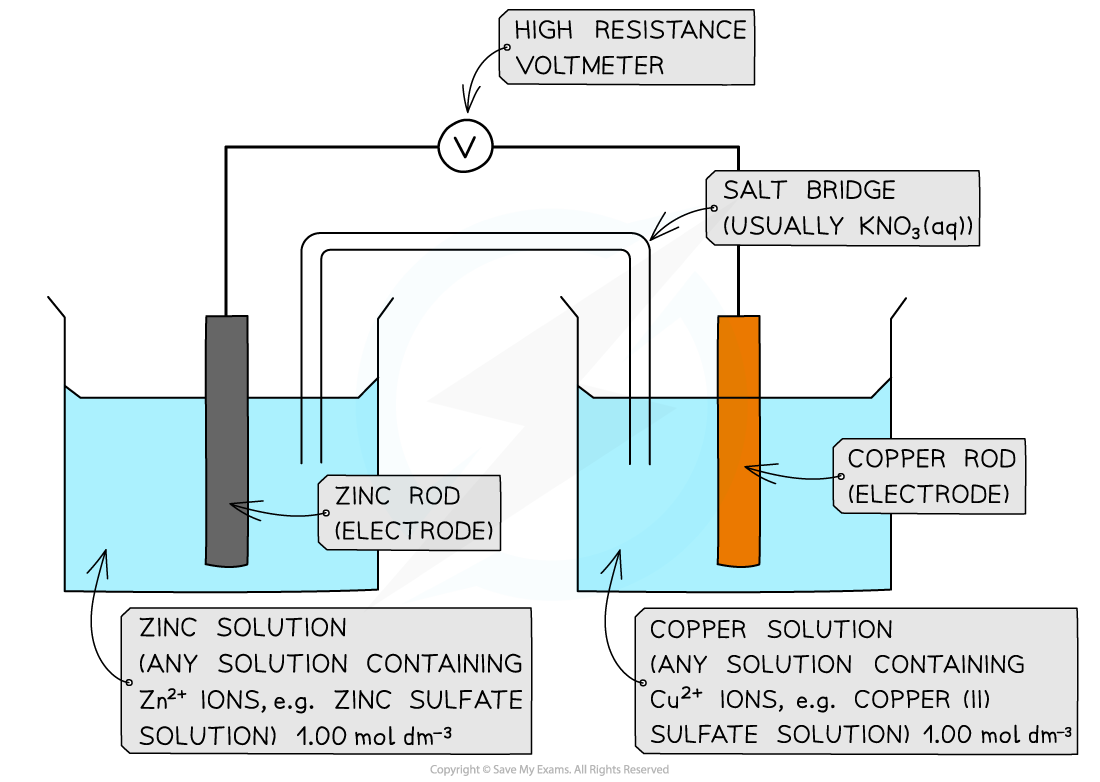
Answer
- Step 1: Calculate the standard cell potential
Ecellꝋ = (+0.34) - (-0.76)
= +1.10 V
The voltmeter will therefore read off a value of 1.10 V
- Step 2: Determine the positive and negative polesThe Cu2+/Cu half-cell is the positive pole as its Eꝋ is more positive than the Eꝋ value of the Zn2+/Zn half-cell
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









