- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记5.3.1 Electrolysis
Products of Electrolysis
- Electrolysis is the breaking down of a compound into its elements using an electric current
- For example, the electrolysis of zinc chloride (ZnCl2) into its elements zinc and chlorine
ZnCl2(s) → Zn(s) + Cl2(g)
- This method is often used to:
- Extract metals from their metal ores when the metals cannot be extracted by heating their ores with carbon
- Purify metals
- Produce non-metals such as fluorine
- Electrolysis is carried out in an electrolysis cell which consists of:
- An electrolyte - this is the compound that is broken down during electrolysis and it is either a molten ionic compound or a concentrated aqueous solution of ions
- Two electrodes - these are metal or graphite rods conduct electricity to the electrolyte and away from the electrolyte
- The positive electrode is called the anode
- The negative electrode is called the cathode
- The power supply, which is direct current
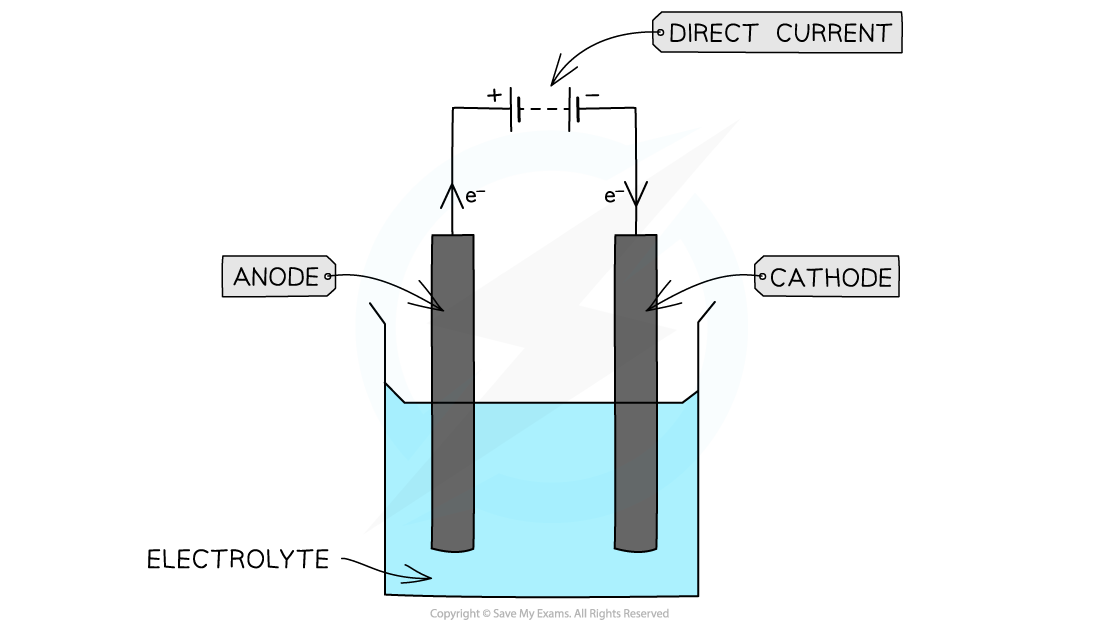
Electrolysis takes place in an electrochemical cell;
this type of electrochemical cell is called an electrolytic cell
Electrolysis of molten electrolytes
- Cations (positively charged ions) move to the negatively charged cathode where they gain electrons
- Reduction takes place at the cathode
- If a metal is formed, a layer of metal is deposited on a cathode or it forms a molten layer in the cell
- If hydrogen gas is formed, bubbles are seen
- For example, silver and hydrogen both form positively charged ions which would be reduced at the cathode as follows:
Ag+ + e- → Ag
2H+ + 2e- → H2
- Anions (negatively charged ions) move to the positively charged anode where they lose electrons
- Oxidation takes place at the anode
- For example, bromine forms negatively charged ions which would be oxidised at the anode as follows:
2Br- → Br2 + 2e-
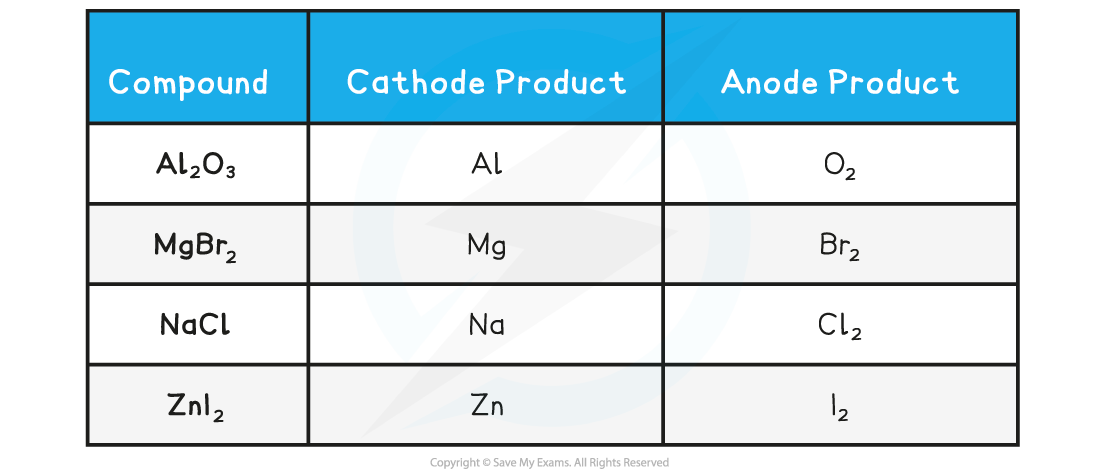
Products formed by electrolysis when a pure molten ionic compound containing two simple ions is electrolysed table
Electrolysis of aqueous solutions
- Aqueous solutions have more than one cation and anion in solution due to the presence of water
- Water contributes H+ and OH- ions to the solution, which makes things more complicated
- Water is a weak electrolyte and splits into H+ and OH- ions as follows:
H2O ⇌ H+ + OH-
- The actual ions that are discharged during electrolysis will depend on:
- The relative electrode potential of the ions
- The concentration of the ions
Relative electrode potential of ions
- The relative electrode potential (Eꝋ) of ions describes how easily an ion is discharged during electrolysis
- The positively charged cation with the most positive Eꝋ will be discharged at the cathode as this is the cation that is most easily reduced
- For example, a concentrated aqueous solution of NaF will contain hydrogen (H+) and sodium (Na+) ions
- The half-equations for the reduction of these ions and their Eꝋ values are as follows:
2H+(aq) + 2e- ⇌ H2(g) Eꝋ = 0.00 V
Na+(aq) + e- ⇌ Na(s) Eꝋ = -2.71 V
- Since H+ ions have a higher Eꝋ value, hydrogen gas (H2) is formed at the cathode instead of sodium (Na)
- The negatively charged anion with the most negative Eꝋ will be discharged at the anode, as this is the anion that is most easily oxidised
- For example, a concentrated aqueous solution of NaF will contain hydroxide (OH-) and fluoride (F-) ions
- The half-equations for the oxidation of these ions and their Eꝋ values are as follows:
4OH-(aq) → O2(g) + 2H2O(l) + 4e- Eꝋ = -0.40 V
2F-(aq) → F2(g) + 2e- Eꝋ = -2.87 V
- Since F- ions have a lower Eꝋ value than OH- ions, fluorine (F2) gas is formed at the anode
Concentration of ions
- Ions that are present in higher concentrations are more likely to be discharged
- For example, when a concentrated solution of NaF is electrolysed, there are far more fluoride ions which are discharged at the anode, instead of the hydroxide ions as the fluoride ions are in higher concentration
- So, mainly fluorine will form at the electrode
- However, if a very dilute solution of NaF is electrolysed, there will be much more oxygen and much less fluorine gas formed at the anode
- In reality, a mixture of both oxygen and fluorine gas is formed
Exam Tip
Electrolysis is a redox reaction as a reduction reaction takes place at one electrode and an oxidation reaction at the other electrode.When writing the overall redox equation make sure that the electrons lost at the anode balance the electrons gained at the cathode.
- Cathode: Cu2+ + 2e- → Cu
- Anode: 2Cl- → Cl2 + 2e-
- Overall: CuCl2 → Cu + Cl2
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








