- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记5.2.5 Reaction Feasibility: Temperature Changes
Feasibility & Temperature Changes
- The feasibility of a reaction can be affected by the temperature
- The Gibbs equation will be used to explain what will affect the feasibility of a reaction for exothermic and endothermic reactions

Exothermic reactions
- In exothermic reactions, ΔHreactionꝋ is negative
- If the ΔSsystemꝋ is positive:
- Both the first and second term will be negative
- Resulting in a negative ΔGꝋ so the reaction is feasible
- Therefore, regardless of the temperature, an exothermic reaction with a positive ΔSsystemꝋ will always be feasible
- If the ΔSsystemꝋ is negative:
- The first term is negative and the second term is positive
- At high temperatures, the -TΔSsystemꝋ will be very large and positive and will overcome ΔHreactionꝋ
- Therefore, at high temperatures ΔGꝋ is positive and the reaction is not feasible
- The reaction is more feasible at low temperatures, as the second term will not be large enough to overcome ΔHreactionꝋ resulting in a negative ΔGꝋ
- This corresponds to Le Chatelier’s principle which states that for exothermic reactions an increase in temperature will cause the equilibrium to shift position in favour of the reactants, i.e. in the endothermic direction
- In other words, for exothermic reactions, the products will not be formed at high temperatures
- The reaction is not feasible at high temperatures
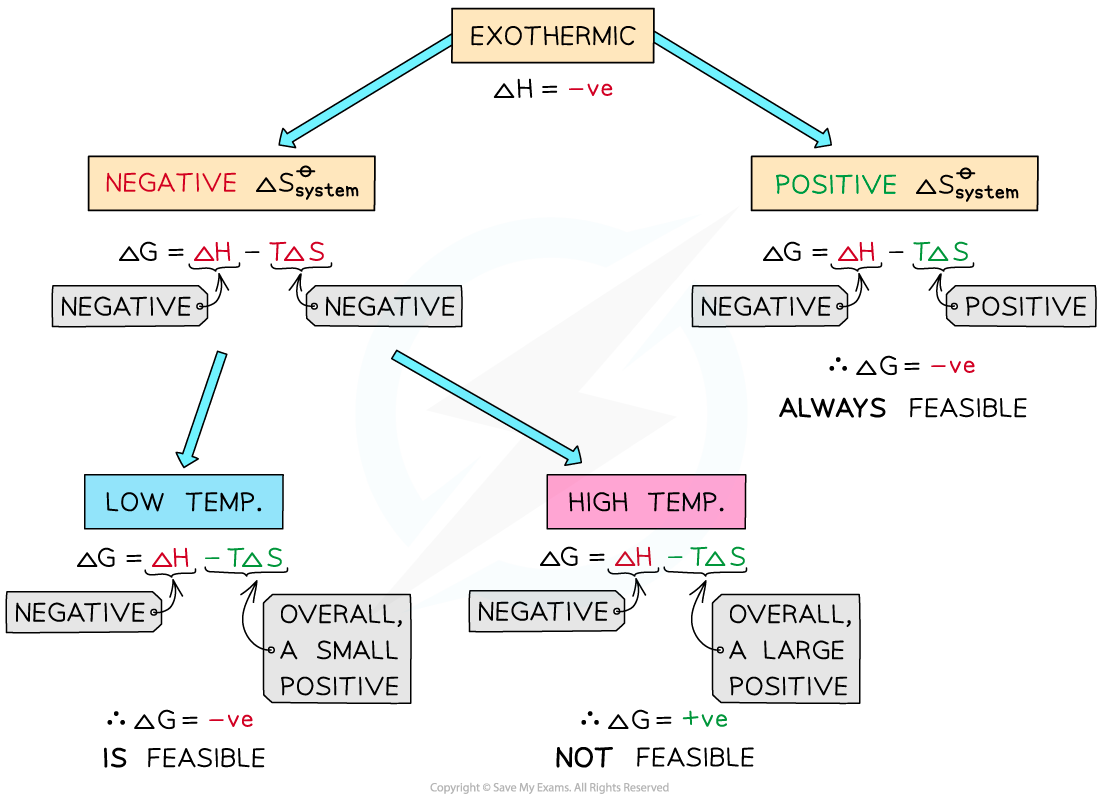
The diagram shows under which conditions exothermic reactions are feasible
Endothermic reactions
- In endothermic reactions, ΔHreactionꝋ is positive
- If the ΔSsystemꝋ is negative:
- Both the first and second term will be positive
- Resulting in a positive ΔGꝋ so the reaction is not feasible
- Therefore, regardless of the temperature, endothermic with a negative ΔSsystemꝋ will never be feasible
- If the ΔSsystemꝋ is positive:
- The first term is positive and the second term is negative
- At low temperatures, the -TΔSsystemꝋ will be small and negative and will not overcome the larger ΔHreactionꝋ
- Therefore, at low temperatures ΔGꝋ is positive and the reaction is less feasible
- The reaction is more feasible at high temperatures as the second term will become negative enough to overcome the ΔHreactionꝋ resulting in a negative ΔGꝋ
- This again corresponds to Le Chatelier’s principle which states that for endothermic reactions an increase in temperature will cause the equilibrium to shift position in favour of the products
- In other words, for endothermic reactions, the products will be formed at high temperatures
- The reaction is therefore feasible
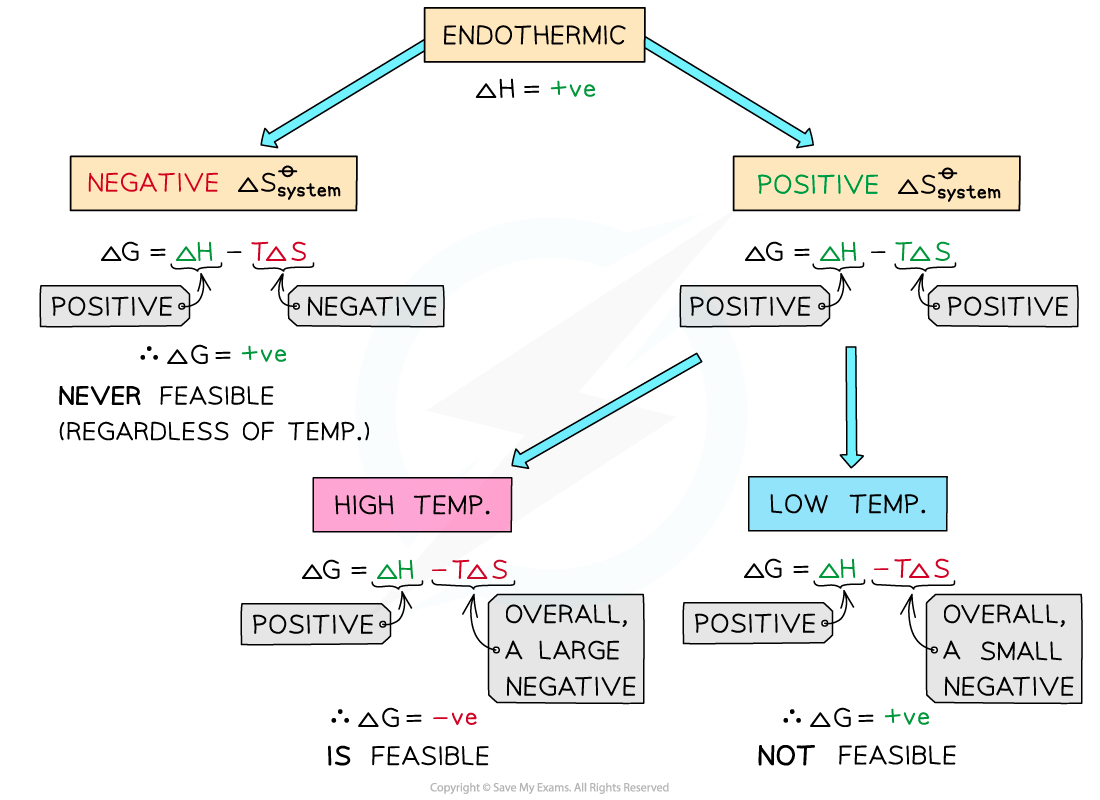
The diagram shows under which conditions endothermic reactions are feasible
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








