- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记5.2.4 Reaction Feasibility
The Gibbs Equation: Reaction Feasibility
- The Gibbs equation can be used to calculate whether a reaction is feasible or not
ΔGꝋ = ΔHreactionꝋ - TΔSsystemꝋ
- When ΔGꝋ is negative, the reaction is feasible and likely to occur
- When ΔGꝋis positive, the reaction is not feasible and unlikely to occur
Worked Example
Worked example: Determining the feasibility of a reaction

Answer
- Step 1: Calculate ΔSsystemꝋ
ΔSsystemꝋ = ΣΔSproductsꝋ - ΣΔSreactantsꝋ
ΔSsystemꝋ = (2 x ΔSꝋ [CaO(s)]) - (2 x ΔSꝋ [Ca(s)] + ΔSꝋ [O2(g)])
= (2 x 40.00) - (2 x 41.00 + 205.0)
= -207.0 J K-1 mol-1
- Step 2: Convert ΔSꝋ to kJ K-1 mol-1
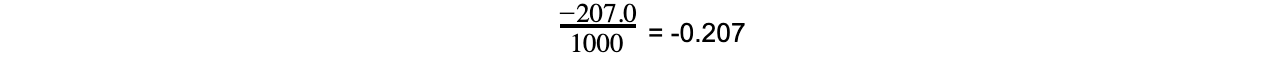
= -0.207
- Step 3: Calculate ΔGꝋ
ΔGꝋ = ΔHreactionꝋ - TΔSsystemꝋ
= -635.5 - (298 x -0.207)
= -573.8 kJ mol-1
- Step 4: Determine whether the reaction is feasibleSince the ΔGꝋ is negative the reaction is feasible and likely to occur
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









