- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记5.2.3 Gibbs Free Energy Change & Gibbs Equation
The Gibbs Equation
Gibbs free energy
- The feasibility of a reaction does not only depend on the entropy change of the reaction, but can also be affected by the enthalpy change
- Therefore, using the entropy change of a reaction only to determine the feasibility of a reaction is inaccurate
- The Gibbs free energy (G) is the energy change that takes into account both the entropy change of a reaction and the enthalpy change
- The Gibbs equation is:
ΔGꝋ = ΔHreactionꝋ - TΔSsystemꝋ
- The units of ΔGꝋ are in kJ mol-1
- The units of ΔHreactionꝋ are in kJ mol-1
- The units of T are in K
- The units of ΔSsystemꝋ are in J K-1 mol-1 (and must therefore be converted to kJ K-1 mol-1 by dividing by 1000)
The Gibbs Equation: Calculations
- The Gibbs equation can be used to calculate the Gibbs free energy change of a reaction
ΔGꝋ = ΔHreactionꝋ - TΔSsystemꝋ
- The equation can also be rearranged to find values of ΔHreactionꝋ, ΔSsystemꝋ or the temperature, T
- For example, if for a given reaction, the values of ΔGꝋ, ΔHreactionꝋ and ΔSsystemꝋ are given, the temperature can be found by rearranging the Gibbs equation as follows:
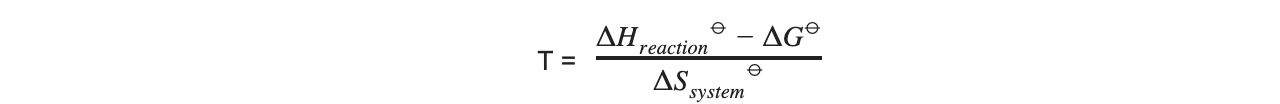
Worked example: Calculating Gibbs free energy
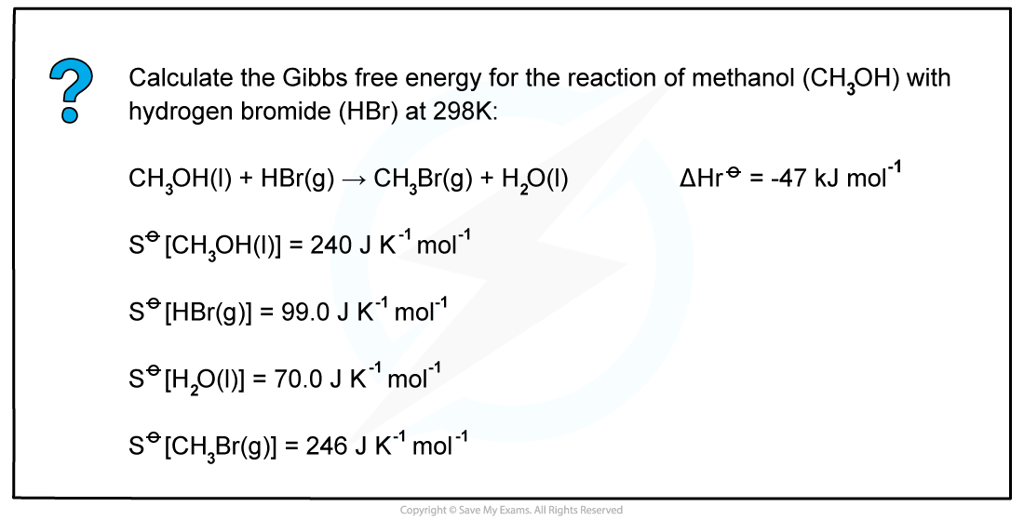
Answer
- Step 1 - Calculate ΔSsystemꝋ
ΔSsystemꝋ = ΣΔSproductsꝋ - ΣΔSreactantsꝋ
ΔSsystemꝋ = (ΔSꝋ [CH3Br(g)] + ΔSꝋ [H2O(l)]) - (ΔSꝋ [CH3OH(l)] + ΔSꝋ[HBr(g)])
= (246 + 70.0) - (240 + 99.0)
= -23.0 J K-1 mol-1
- Step 2 - Convert ΔSꝋ into kJ K-1 mol-1
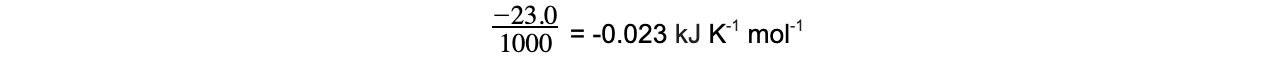
= -0.023 kJ K-1 mol-1
- Step 3 - Calculate ΔGꝋ
ΔGꝋ = ΔHreactionꝋ - TΔSsystemꝋ
= -47 - (298 x -0.023)
= -40.146 kJ mol-1
= -40.1 kJ mol-1
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









