- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记5.2.2 Calculating Entropy Changes
Calculating Entropy Changes
- The standard entropy change (ΔSsystemꝋ ) for a given reaction can be calculated using the standard entropies (Sꝋ ) of the reactants and products
- The equation to calculate the standard entropy change of a system is:
ΔSsystemꝋ = ΣΔSproductsꝋ - ΣΔSreactantsꝋ
(where Σ = sum of)
- For example, the standard entropy change for the formation of ammonia (NH3) from nitrogen (N2) and hydrogen (H2) can be calculated using this equation
N2(g) + 3H2(g) ⇋ 2NH3(g)
ΔSsystemꝋ = (2 x ΔSꝋ(NH3)) - (ΔSꝋ(N2) + 3 x ΔSꝋ(H2))
Worked example: Calculating entropy changes
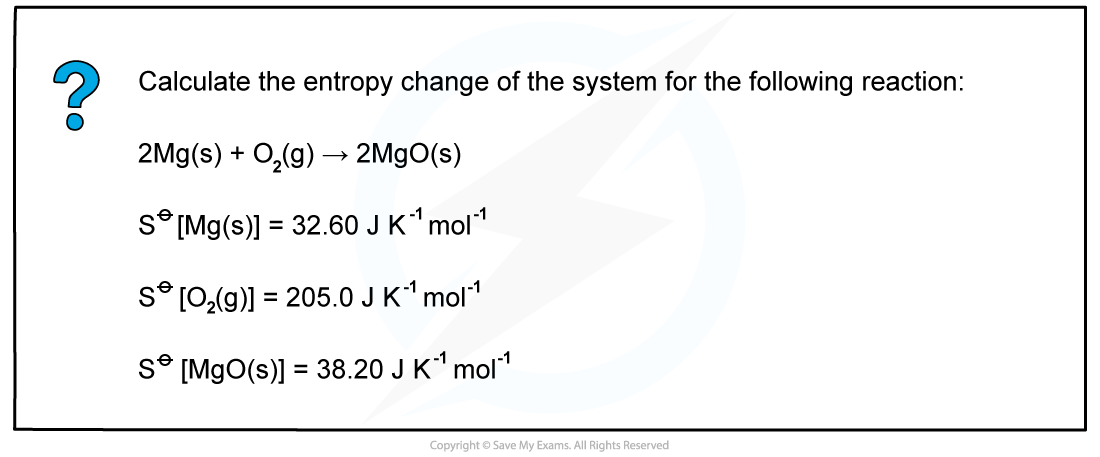
Answer
ΔSsystemꝋ = ΣΔSproductsꝋ - ΣΔSreactantsꝋ
ΔSsystemꝋ = (2 x 38.20) - (2 x 32.60 + 205.0)
= -193.8 J K-1 mol-1
Exam Tip
Use the stoichiometry of the equation and the correct state of the compounds when calculating the entropy change of a reaction.
转载自savemyexams


最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








