- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记5.2.1 Entropy & Entropy Change
Defining Entropy
- The entropy (S) of a given system is the number of possible arrangements of the particles and their energy in a given system
- In other words, it is a measure of how disordered a system is
- When a system becomes more disordered, its entropy will increase
- An increase in entropy means that the system becomes energetically more stable
- For example, during the thermal decomposition of calcium carbonate (CaCO3) the entropy of the system increases:
CaCO3(s) → CaO(s) + CO2(g)
- In this decomposition reaction, a gas molecule (CO2) is formed
- The CO2 gas molecule is more disordered than the solid reactant (CaCO3), as it is constantly moving around
- As a result, the system has become more disordered and there is an increase in entropy
- Another typical example of a system that becomes more disordered is when a solid is melted
- For example, melting ice to form liquid water:
H2O(s) → H2O(l)
- The water molecules in ice are in fixed positions and can only vibrate about those positions
- In the liquid state, the particles are still quite close together but are arranged more randomly, in that they can move around each other
- Water molecules in the liquid state are therefore more disordered
- Thus, for a given substance, the entropy increases when its solid form melts into a liquid
- In both examples, the system with the higher entropy will be energetically the most stable (as the energy of the system is more spread out when it is in a disordered state)
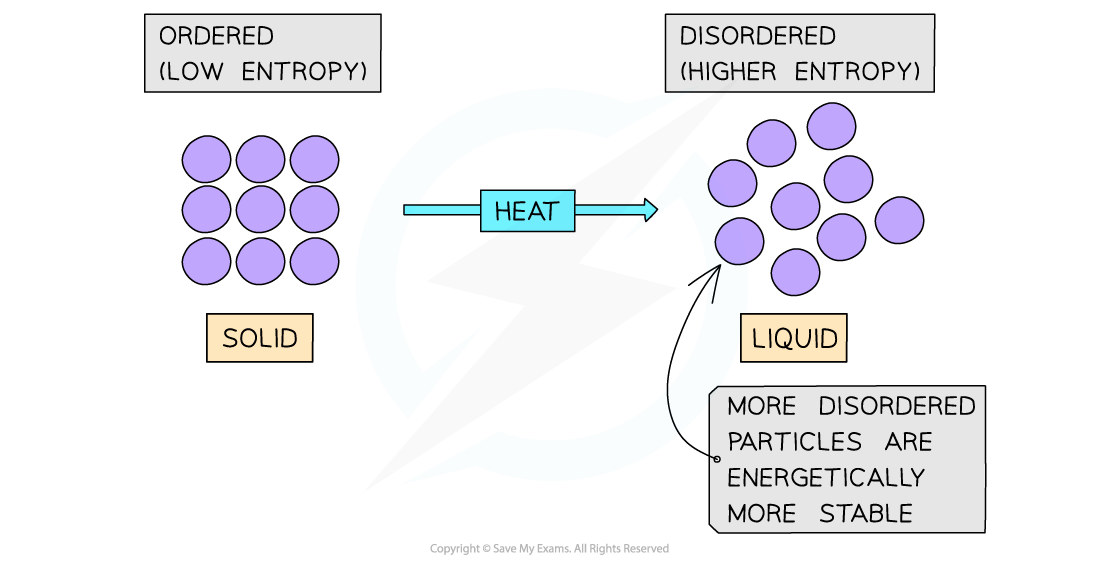
Melting a solid will cause the particles to become more disordered resulting in a more energetically stable system
Exam Tip
Make sure you don’t confuse the system with your surroundings!The system consists of the molecules that are reacting in a chemical reaction.The surroundings are everything else such as the solvent, the air around the reaction, test-tube, etc.
Entropy Changes
- All elements have positive standard molar entropy values
- The order of entropy for the different states of matter are as follows:
gas > liquid > solid
-
- There are some exceptions such as calcium carbonate (solid) which has a higher entropy than mercury (liquid)
- Simpler substances with fewer atoms have lower entropy values than complex substances with more atoms
- For example, calcium oxide (CaO) has a smaller entropy than calcium carbonate (CaCO3)
- Harder substances have lower entropy than softer substances of the same type
- For example, diamond has a smaller entropy than graphite
Change in state
- The entropy of a substance changes during a change in state
- The entropy increases when a substance melts (change from solid to liquid)
- Increasing the temperature of a solid causes the particles to vibrate more
- The regularly arranged lattice of particles changes into an irregular arrangement of particles
- These particles are still close to each other but can now rotate and slide over each other in the liquid
- As a result, there is an increase in disorder
- The entropy increases when a substance boils (change from liquid to gas)
- The particles in a gas can now freely move around and are far apart from each other
- The entropy increases significantly as the particles become very disordered
- Similarly, the entropy decreases when a substance condenses (change from gas to liquid) or freezes (change from liquid to solid)
- The particles are brought together and get arranged in a more regular arrangement
- The ability of the particles to move decreases as the particles become more ordered
- There are fewer ways of arranging the energy so the entropy decreases
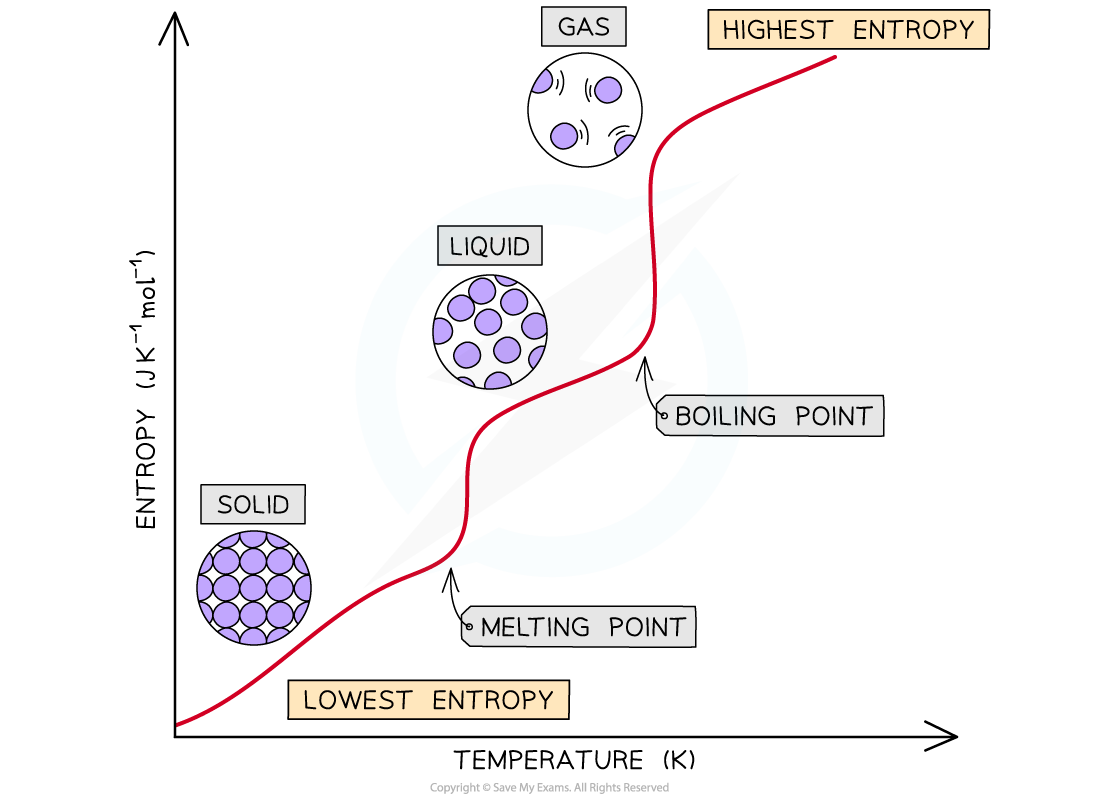
The entropy of a substance increases when the temperature is raised as particles become more disordered
- The entropy also increases when a solid is dissolved in a solvent
- The solid particles are more ordered in the solid lattice as they can only slightly vibrate
- When dissolved to form a dilute solution, the entropy increases as:
- The particles are more spread out
- There is an increase in the number of ways of arranging the energy
- The crystallisation of a salt from a solution is associated with a decrease in entropy
- The particles are spread out in solution but become more ordered in the solid
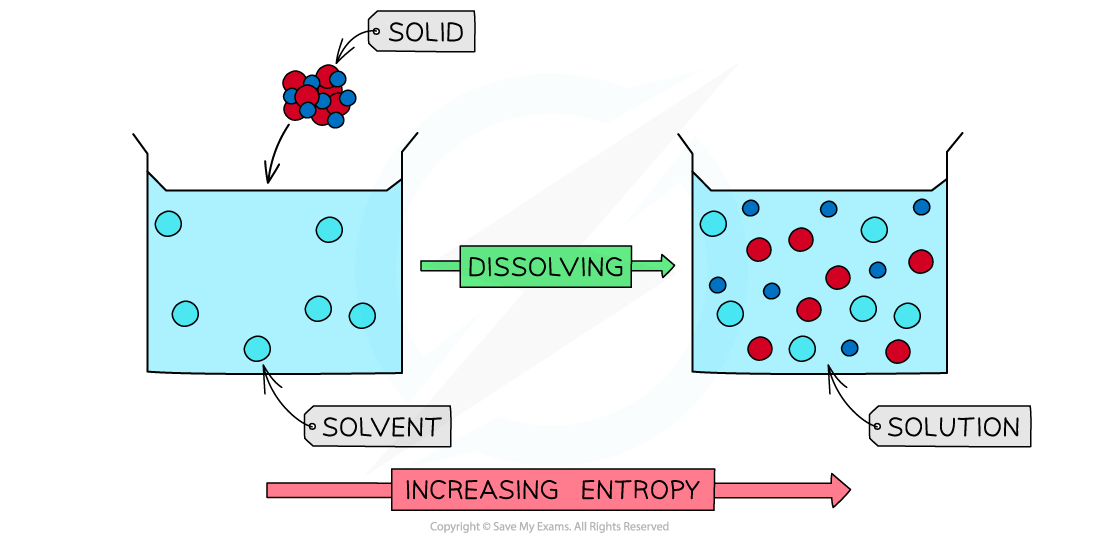
When a solid is dissolved in a solvent to form a dilute solution, the entropy increases as the particles become more disordered
Entropy changes in reactions
- Gases have higher entropy values than solids
- So, if the number of gaseous molecules in a reaction changes, there will also be a change in entropy
- The greater the number of gas molecules, the greater the number of ways of arranging them, and thus the greater the entropy
- For example the decomposition of calcium carbonate (CaCO3)
CaCO3(s) → CaO(s) + CO2(g)
-
- The CO2 gas molecule is more disordered than the solid reactant (CaCO3) as it can freely move around whereas the particles in CaCO3 are in fixed positions in which they can only slightly vibrate
- The system has therefore become more disordered and there is an increase in entropy
- Similarly, a decrease in the number of gas molecules results in a decrease in entropy causing the system to become less energetically stable
- For example, the formation of ammonia in the Haber process
N2(g) + 3H2(g) ⇋ 2NH3(g)
-
- In this case, all of the reactants and products are gases
- Before the reaction occurs, there are four gas molecules (1 nitrogen and 3 hydrogen molecules) in the reactants
- After the reaction has taken place, there are now only two gas molecules (2 ammonia molecules) in the products
- Since there are fewer molecules of gas in the products, there are fewer ways of arranging the energy of the system over the products
- The system has become more ordered causing a decrease in entropy
- The reactants (N2 and H2) are energetically more stable than the product (NH3)
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









