- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记5.1.9 Factors Affecting Enthalpy of Hydration
Enthalpy of Hydration: Ionic Charge & Radius
- The standard enthalpy change of hydration (ΔHhydꝋ) is affected by the amount that the ions are attracted to the water molecules
- The factors which affect this attraction are the ionic charge and radius
Ionic radius
- ΔHhydꝋ becomes more exothermic with decreasing ionic radii
- Smaller ions have a greater charge density resulting in stronger ion-dipole attractions between the water molecules and the ions in the solution
- Therefore, more energy is released when they become hydrated and ΔHhydꝋ becomes more exothermic
- For example, the ΔHhydꝋ of magnesium sulfate (MgSO4) is more exothermic than the ΔHhydꝋ of barium sulfate (BaSO4)
- Since both compounds contain a sulfate (SO42-) ion, the difference in ΔHhydꝋ must be due to the magnesium (Mg2+) ion in MgSO4 and barium (Ba2+) ion in BaSO4
- Magnesium is a Group 2 and Period 3 element
- Barium is a Group 2 and Period 6 element
- This means that the Mg2+ ion is smaller than the Ba2+ ion
- The attraction is therefore much stronger for the Mg2+ ion
- As a result, the standard enthalpy of hydration of MgSO4 is more exothermic than that of BaSO4
Ionic charge
- ΔHhydꝋ is more exothermic for ions with larger ionic charges
- Ions with large ionic charges have a greater charge density resulting in stronger ion-dipole attractions between the water molecules and the ions in the solution
- Therefore, more energy is released when they become hydrated and ΔHhydꝋ becomes more exothermic
- For example, the ΔHhydꝋ of calcium oxide (CaO) is more exothermic than the ΔHhydꝋ of potassium chloride (KCl)
- Calcium oxide is an ionic compound that consists of calcium (Ca2+) and oxide (O2-) ions
- Potassium chloride is formed from potassium (K+) and chloride (Cl-) ions
- Both of the ions in calcium oxide have a greater ionic charge than the ions in potassium chloride
- This means that the attractions are stronger between the water molecules and Ca2+ and O2- ions upon hydration of CaO
- The attractions are weaker between the water molecules and K+ and Cl- ions upon hydration of KCl
- Therefore, the ΔHhydꝋ of calcium oxide is more exothermic as more energy is released upon its hydration
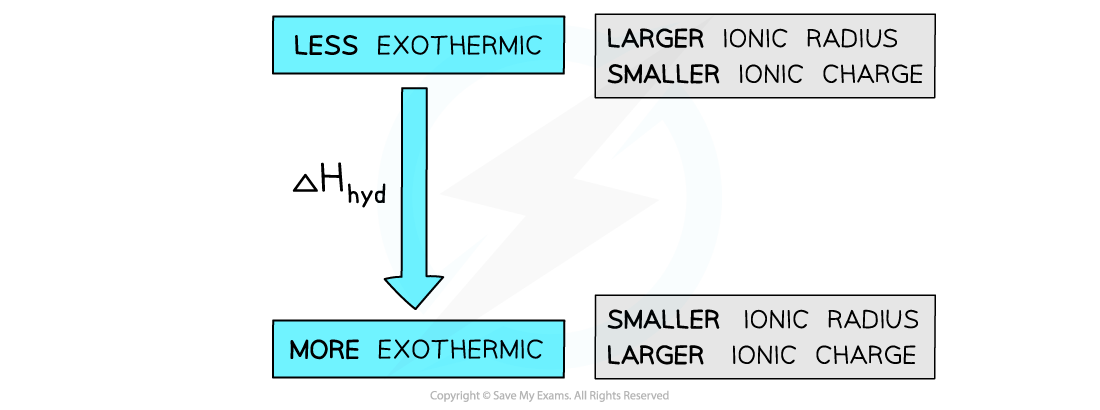
The enthalpy of hydration is more exothermic for smaller ions and for ions with a greater ionic charge
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








